OK Rapid HIV Testing Out of Labs
- Share via
There was a major victory in the battle against HIV/AIDS recently when the Food and Drug Administration approved the first easy-to-use “rapid” test for HIV, called OraQuick. This test provides results in about 20 minutes instead of the two days to two weeks required with standard HIV tests.
Unfortunately, the FDA approved the test for use only in facilities with certified laboratories. This requirement excludes OraQuick testing in mobile vans and at other sites that serve low-income and uninsured populations.
Can the rapid test be safely conducted in sites without a certified laboratory, such as mobile vans? The rapid test, available in more than 90 countries, is no more difficult to use than at-home glucose tests for diabetes.
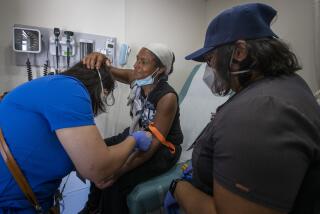
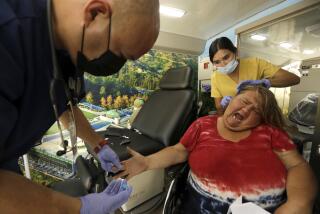
To conduct the OraQuick HIV test, a person pricks his or her finger and places a drop of blood in a vial, mixing it with a solution. The test kit is then inserted into the vial. In 20 minutes or less, two reddish-purple lines in the test’s window will indicate whether the blood is infected with the HIV virus.
The test is remarkably reliable, with very small percentages of false negative or false positive results. Nonetheless, any positive test is confirmed with the standard tests that are currently in use.
Los Angeles County provides free and convenient HIV testing in mobile vans for those who are at significant risk. But under the current system, clients served by these vans must return at a later date to receive their HIV test results.
Results of a 1999 county survey found that 25% of those who obtained their HIV test in a mobile medical van did not return for their results. In contrast, 9% of those who were tested in their doctor’s office and 11% of those who were tested in family planning or community clinics failed to learn the results of their HIV test.
The percentage of clients receiving results would be greatly increased if it were possible to use the OraQuick test to provide results on the spot.
The benefits of rapid testing extend to other nontraditional sites for health-care delivery, such as county jails. The transient nature of the jail population makes it imperative to provide results as quickly as possible to the tested inmates.
It is essential that people with HIV learn their status early so they can receive drugs to delay the onset of AIDS. Further, it is important that testing be accompanied by counseling to assist clients in reducing risky behavior.
Recognizing the importance of counseling and testing, the federal Centers for Disease Control and Prevention has established testing to promote early diagnosis as one of the three elements in a strategic plan to reduce annual HIV infections by half within five years. The rapid test will probably be less costly, further enhancing the CDC’s efforts to increase testing.
The FDA should approve the manufacturer’s application to waive restrictions limiting use of the OraQuick rapid test to laboratories. That federal agency’s approval last month of three at-home glucose tests for diabetes provides a relevant and timely precedent for granting such a waiver.
The many positive benefits of early identification of people who are HIV-positive argue for dissemination of the rapid-test technology to mobile vans and other sites without a certified laboratory.
Informing those who are HIV-positive of their status not only allows them to begin early treatment with effective antiretroviral medications, it can result in reduction of behavior that carries risk of transmitting HIV.
*
Arleen Leibowitz is a professor of policy studies at the UCLA School of Public Policy and Social Research and a member of the UCLA Center for HIV Identification, Prevention and Treatment Services, where Stephanie Taylor is a fellow. Jonathan Fielding, the health officer for Los Angeles County, is a pediatrics professor at UCLA.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.