SLIPPERY CHARACTERS
- Share via
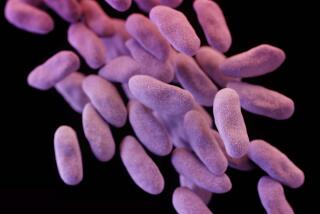
WHETHER on a contact lens or a catheter, in your lungs or in your ears, a few bacterial invaders can set up a slimy fortress that can prove almost impossible to demolish. All it takes is a wet surface and a few days.
The sticky mess is called a biofilm, a slick layer of bacteria that is one of the biggest problems facing medicine today. Biofilms are forcing scientists to reevaluate their view of bacteria as free-floating bugs to one of sophisticated communities stuck on surfaces.
The National Institutes of Health estimates that more than 80% of microbial infections in the human body are caused by biofilms, many of them creating chronic and reoccurring problems. Two of the most serious: The layer of Pseudomonas aeruginosa that forms in the trachea of cystic fibrosis patients and the hospital-acquired infections resulting from biofilm formation on implanted medical devices.
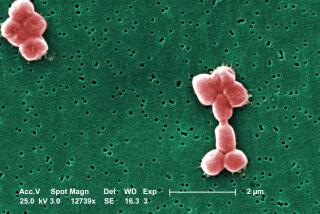
Even the healthiest among us deals with biofilms on a daily basis in the form of dental plaque. And biofilms containing a pathogenic strain of E. coli were behind the spinach recall in October.
The problem with biofilms is that, because of their tightknit structure, they are resistant to traditional disinfectants and antibiotics. New research is aimed at finding ways to battle these goo-covered conglomerates. By understanding how biofilms thrive, scientists are devising new strategies for defeating them.
Biofilms form in a series of steps that scientists are just starting to understand. Step one occurs when a few bacteria attach to a surface. They activate certain genes within their genomes and inactivate others, starting their transformation from free-floaters to biofilm.
The bacteria begin to secrete polymers that hold aggregates of cells together. Then, as the biofilm grows, it becomes more complex and even starts to act like a multicellular organism.
Structures arise -- such as channels to bring in nutrients and take away wastes.
Bacteria in different areas of the biofilm take on different roles. Some cells secrete enzymes, while others continue to make sticky matrix proteins. Some bacteria continue to rapidly divide, while others enter a dormant state.
The heterogeneity of biofilms is part of what makes them so robust, says Phil Stewart, director of the Center for Biofilm Engineering at Montana State University in Bozeman. A drug that can kill some of the bacteria in the biofilm might be useless against some of their neighbors.
The final step in the biofilm life cycle is dispersion. As conditions get crowded and resources become scarce, groups of cells detach and float away -- potentially setting up new biofilms if they happen to land in a good spot.
“It’s a little bit like seeds,” Stewart says.
In many chronic and recurring infections -- such as ear infections, prostatitis in men, urinary tract infections and endocarditis -- these biofilm seeds set off the immune system and cause symptoms such as fever and inflammation that signal to doctor and patient that something’s amiss.
When antibiotics are administered, the free-floating bacteria are killed. But the original biofilm remains unscathed. Although the infection appears to be cured, it is only a matter of time before another chunk of biofilm is set free and the symptoms return.
There are no good ways to fight biofilms because an awareness of their importance has only emerged in the last decade, Stewart says. Before that, research on bacteria focused on homogeneous cultures of fast-growing cells in flasks. Tools that fight these kinds of bacteria are great for acute infections in which bacteria are freely floating in the body. But they are almost useless against biofilms.
The Center for Biofilm Engineering is trying to change this by developing standardized methods for working with biofilms that the whole research community can use. A few companies and academic scientists are already exploring new avenues in treating and preventing biofilms.
One of the problems with today’s antibiotics is that many only attack bacteria that are actively growing. This means that the dormant cells in a biofilm will escape treatment and be left behind to regrow the biofilm. So one approach to fighting biofilms is to come up with drugs that can kill all the biofilm residents.
Cubist Pharmaceuticals in Lexington, Mass., developed an antibiotic called daptomycin (marketed as Cubicin) that is able to do just that, enabling doctors to eradicate a biofilm with drug treatment.
But even if you could use antibiotics to kill all the bacteria in a biofilm, that might not be the best idea, says Wenyuan Shi, professor of oral biology and medicine at UCLA’s School of Dentistry.
“Think of a lawn infested with dandelions,” he says. “If you kill everything, the dandelions will come back first. But if you use a dandelion-specific killer and the grass fills in the lawn, the dandelions won’t come back.”
Many biofilms are composed of both good and bad bacteria. Shi’s lab has developed a “smart bomb” that targets the harmful bacteria in a biofilm. A short antimicrobial peptide is attached to another one that mimics a pheromone used by bacteria to communicate. This hybrid bacteria-fighting molecule attaches to certain bacteria and makes sure only the targeted bugs are killed.
Shi has formed a Los Angeles-based company, C3Jian, to develop this technique for use in dental hygiene products. The company recently received the go-ahead for a clinical pilot study of a dental varnish that incorporates its peptides. It expects to have a product on the market in about three years.
Another approach to fighting biofilms is to try to break up their slimy coating and return them to the free-floating state that scientists are used to dealing with. Once released from the biofilm, the bacteria can be killed with antibiotics.
Kane Biotech, a company based in Winnipeg, Canada, is developing treatments and preventives based on an enzyme called Dispersin B, which was discovered in the bacterial species Actinobacillus actinomycetemcomitans but is now mass produced by the company using E. coli bacteria. Dispersin B breaks up the sugary bonds in the slime of biofilms, separating the bacteria from one another.
In animal studies, the company has reported that a combination of Dispersin B and broad-spectrum antibiotics can effectively prevent biofilm buildup in central venous catheters. (Such catheters, which are inserted into large veins in the neck, chest or groin, frequently become colonized by biofilms when bacteria make their way in from the patients’ skin or from contact with healthcare workers.) Kane is also working on ways to incorporate Dispersin B into treatments for chronic wounds such as diabetic foot ulcers, which are thought to be slow-healing because of infections by biofilms.
David Davies, assistant professor of biological sciences at Binghamton University in New York, also is taking the divide-and-conquer approach. But instead of attacking biofilm slime from the outside with an enzyme, his lab is working with a chemical messenger derived from biofilms themselves.
This messenger -- its name is proprietary -- is common to many species of biofilm bacteria and is responsible for the dispersion effect that is a normal part of their life cycle. Davies’ group intends to tweak this messenger so that it could be used as a medicine that would trick biofilms into thinking it was time to break up. Once they do so, it would make the bacteria susceptible to classical antibiotic treatments.
Biosignal, an Australian biotech company, is trying to prevent biofilms from forming on medical devices in the first place. Its product is derived from a type of seaweed called Delisea pulchra that is found off the Australian coast. Although many surfaces in the ocean are covered with biofilm, the leaves of this plant are not. That’s because they produce chemicals called furanones that prevent biofilm formation.
Furanones don’t kill bacteria. According to Stewart, this is ideal. By not exposing the bacteria to poisons, there is less pressure for the selection of resistant strains -- a problem that has become widespread with the increased use of antibiotics.
Peter Steinberg, a professor of biological sciences at the University of New South Wales in Australia and the head of research and development at Biosignal, says that the company has found a way to produce refined versions of furanones in the lab. The company is using them to make coatings for contact lenses. A small clinical trial in Australia involving 10 patients wearing the coated contact lenses overnight showed that the furanone coating is safe and well-tolerated.
The firm is working toward larger, longer trials and is in the early stages of developing furanone coatings for medical devices such as catheters.
A group in Israel led by Gad Lavie, an assistant professor at Sheba Medical Center, is taking a more physical approach to the biological problem of biofilms on medical devices. Working with NanoVibronix, an Israeli company, Lavie has found that when scientists attach a small acoustic-wave-producing device to the outside portion of a urinary catheter, biofilm formation is prevented.
The acoustic waves cause vibrations in the catheter that are imperceptible to patients but apparently repulsive to bacteria. In a small clinical trial conducted last year in Germany, six of seven patients fitted with nonvibrating catheters in their urethras developed biofilms in them. None of the vibrating catheters showed any signs of biofilm formation.
“It was really amazing,” Lavie says. These results are significant, he adds, because patients who are catheterized for long periods of time must have their catheters changed every four to five days because of biofilm buildup. He expects that vibrating catheters might not need to be replaced for months.
Biofilm research is still an emerging field. Although many approaches for dealing with biofilms in health and industrial settings have been thought up, most are still in the development stage. For now, the surest way to proceed in treating biofilms is to remove them when possible.
In hospitals, this means replacing catheters every few days. In the kitchen, it means really scrubbing produce clean. And, of course, Stewart says, “tooth brushing is a good idea.”
--
(BEGIN TEXT F INFOBOX)
Breeding grounds
Biofilms are not just in the human body. They can occur on any moist surface.
Fresh produce: The moist, nutrient-rich surfaces of fruits and vegetables are prime for biofilms. They can be hard to remove even with washing. This is not usually a problem unless a pathogenic bacterium, such as the strain of E. coli that was involved in the spinach recall, becomes part of the mix. Eco-Safe Systems USA is marketing a product that dissolves ozone gas in water, producing an antibacterial wash that can kill bacteria instantly, even when it is hiding in a biofilm. The ozone then breaks down to oxygen. The USDA allows produce washed with ozonated water to be labeled organic.
Industrial pipes: Biofilms can set up residence inside pipes and cause devastating corrosion. They were behind the breach found in the Alaska Pipeline last summer. Sixteen miles of pipe had to be replaced.
Household pipes: Biofilms can also build up in water pipes and air-conditioning ducts. If they grow in the pipes feeding the hot tubs, chunks of bacteria can break off, enter the hot tub and become aerosolized, leading to an infection known as “hot tub lung.”
-- Erin Cline