Olympus mounts defense over deadly outbreaks tied to medical scopes
- Share via
Reporting from washington — Under fire for selling a medical scope linked to superbug outbreaks, Olympus Corp. is pushing back against its critics and says the design of its product isn’t necessarily the sole cause for infections.
U.S. health officials have determined that recent bacterial outbreaks were tied to reusable duodenoscopes sold by Olympus and two other manufacturers — even though hospitals followed the companies’ cleaning instructions.
Likewise, physicians and researchers have cited a design flaw that makes these gastrointestinal scopes difficult to clean and prone to spreading dangerous bugs from patient to patient. In some cases, hospitals say contaminated scopes have become impossible to clean and never could be used again.
Those design issues and the potential danger to patients can be compounded, experts say, when low-paid, entry-level employees tasked with quickly washing these intricate devices make mistakes or skip important steps.
Olympus executives, in mounting the company’s defense, are emphasizing the potential for human errors.
“There are multiple factors that influence a scope that is safe and patient ready for next use,” said Laura Storms, vice president of regulatory and clinical affairs and quality assurance at Olympus.
“Reprocessing the scope immediately, paying attention to the details, ensuring that the scope is adequately dried,” Storms said. “Endoscopes seem simple, but they are not.”
Olympus didn’t share any of this during a two-day meeting last week of a Food and Drug Administration advisory panel. The company declined to participate, angering some of the doctors on the committee as well as the families of some patients who became infected.
The FDA committee deemed the duodenoscopes unsafe given the infection risk, but agreed the scopes should remain on the market because they’re used in a potentially lifesaving procedure with no better alternative.
On Monday, an FDA official said the agency will be working with manufacturers on the possibility of redesigned scopes or disposable devices. But industry experts say no new products appear likely in the short term, so much of the immediate attention has focused on everyday cleaning techniques.
In response to the outbreaks, Olympus issued revised cleaning instructions in March and began shipping smaller cleaning brushes to customers this month.
Last week at the FDA hearing, some physicians criticized Olympus for not sharing what it knows about the effectiveness of its new cleaning methods.
Storms said it would not have been appropriate for the company to discuss that information because it’s part of a pending application before federal regulators.
Olympus has been selling its TJF-Q180V duodenoscope — without the necessary government clearance — since 2010. At the FDA’s request, the company subsequently filed for that approval.
“Olympus is currently working with the Food and Drug Administration and additional updates may be available soon,” Storms said. “It would be irresponsible for Olympus to release any data at this point.”
But at events this week, company representatives were showing no such restraint in courting doctors and nurses at two large professional conferences in the Washington area.
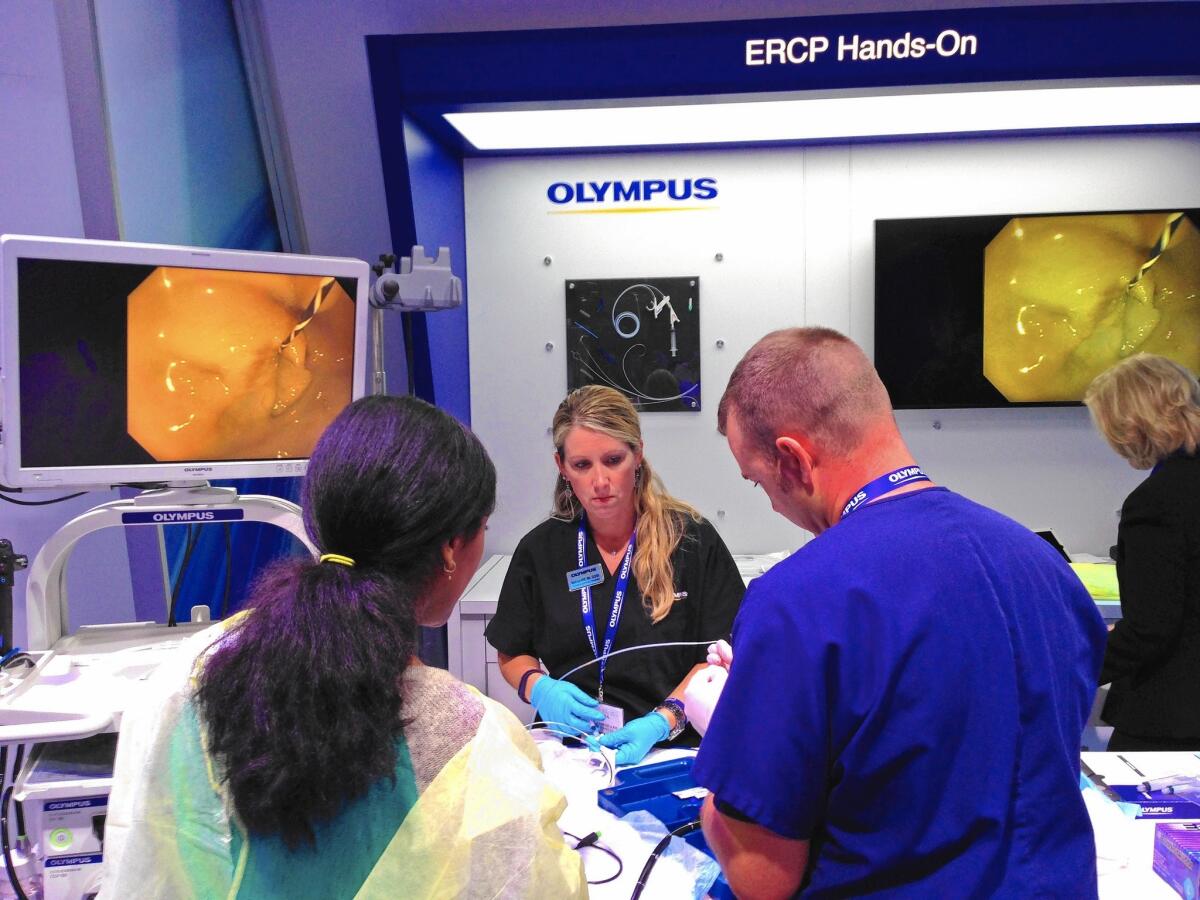
The flashy showcase for Olympus products on the exhibit floor at the Digestive Disease Week conference in Washington attracted hundreds of doctors from across the country and the world.
Physicians crowded in to watch hands-on demonstrations of Olympus equipment and chat with sales representatives.
Nearby, the Olympus logo was posted prominently as the leading sponsor of an educational area run by the American Society for Gastrointestinal Endoscopy where doctors watched instructional videos on procedures.
Dr. Ahmed Badi, a gastroenterologist from Qatar, donned a yellow surgical gown over his gray suit and tried out the Olympus 180V scope on a pig stomach plopped in a blue tray. He fiddled with the scope’s knobs and guided the device down, watching his progress on a large TV screen.
Two Olympus nurses in dark blue scrubs threaded plastic stents and balloons into the scope for the doctor to practice with.
“That’s very good, very good,” Olympus nurse Teddy Thompson told the doctor. “How many [procedures] do you do in a day?”
Badi walked away impressed.
“Olympus is one of the best scopes in the world,” he said, citing its durability and wide number of accessories.
Pentax and Fujifilm, two other duodenoscope manufacturers, had similar exhibits at the physician conference. Doctors were lined up to get free lab coats from Pentax monogrammed with their names. Olympus is the dominant supplier, with an estimated 85% share of the U.S. market for specialty endoscopes.
The duodenoscopes are used in a procedure called ERCP, or endoscopic retrograde cholangiopancreatography. About 670,000 ERCP procedures were performed in the U.S. last year.
At a separate conference in Baltimore, Olympus held a dinner for more than 120 gastroenterology nurses and outlined its new cleaning instructions.
Mary Ann Drosnock, manager of infection control for Olympus, told audience members that meticulous cleaning by them and their co-workers was essential for safeguarding patients.
After her speech, the nurses split up into small groups and Olympus employees showed off the new cleaning brush and how to use it inside the tip of the scope.
Many of the nurses welcomed the hands-on advice, but they said the extra steps might further complicate what can already be an arduous task.
“We know that cross-contamination does occur,” Drosnock told the audience. “At a minimum, this is inconvenient and creates anxiety for patients. At the worst, it can lead to those life-threatening infections. That is why we are here today.”
Twitter: @chadterhune
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.











