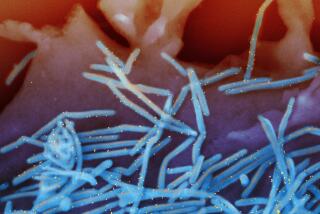
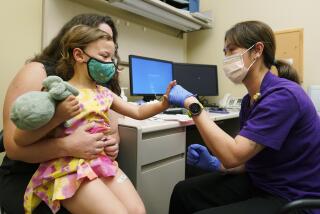
FDA approves meningitis vaccine for 9-month-old infants
- Share via
A meningitis vaccine already in use has been approved by the Food and Drug Administration for children as young as 9 months old.
The two-dose vaccine, Menactra, which produces antibodies against a strain of meningococcus bacteria, was approved in 2005 for 11- to 55-year-olds and in 2007 for 2-year-olds.
Though meningitis cases are relatively rare—about 1,000 to 2,600 cases per year—the disease can be deadly. The FDA announcement states:
“Meningococcal disease is a life-threatening illness caused by bacteria that infect the bloodstream (sepsis) and the lining that surrounds the brain and spinal cord (meningitis) …Even with appropriate antibiotics and intensive care, between 10 percent and 15 percent of people who develop meningococcal disease die from the infection. Another 10 percent to 20 percent suffer complications such as brain damage or loss of limb or hearing.”
Infants younger than 1 year old are most susceptible to the disease, though adults age 18-23 also have higher-than-average infection rates (college students in dorms have an increased risk of contracting the contagious disease).
But the meningitis vaccine isn’t on the list of vaccines generally recommended for young children but rather for those age 11 to 18.
And just because a vaccine is approved in infants doesn’t mean the Centers for Disease Control and Prevention will recommend that it join an immunization list that already includes vaccines against the chicken pox, measles and the flu. But perhaps that’s because the recommendations sometimes take a few years to catch up with new vaccines. Flash back to 2005, when the CDC advisory committee said meningococcal conjugate vaccines, such as Menactra, still needed to stand up to clinical trials:
“Meningococcal conjugate vaccines might be considered for licensing in the United States among persons in other age groups, including infants and children aged <10 years. These vaccines are undergoing clinical trials and are likely to have better immunogenicity among infants and young children than MPSV4, which is the only vaccine available for these age groups in the United States.”
Maybe it’s just a matter of time.