U.S. warns MPX could mutate to resist antiviral drug Tpoxx
- Share via
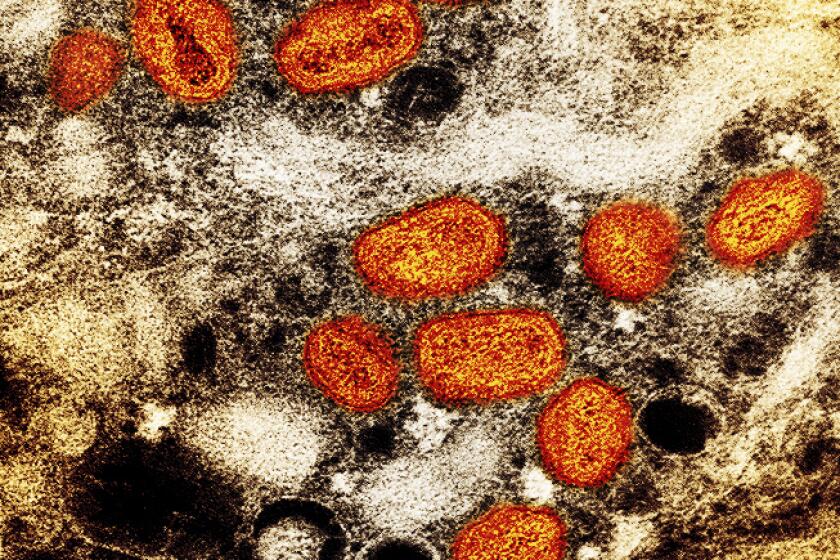
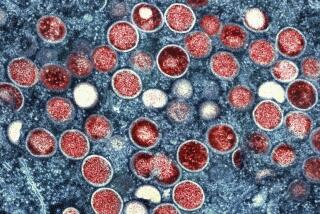
WASHINGTON — U.S. health officials are warning against overuse of the lone drug available to treat monkeypox, or MPX, saying that even a small mutation in the virus that causes the disease could render the pills ineffective.
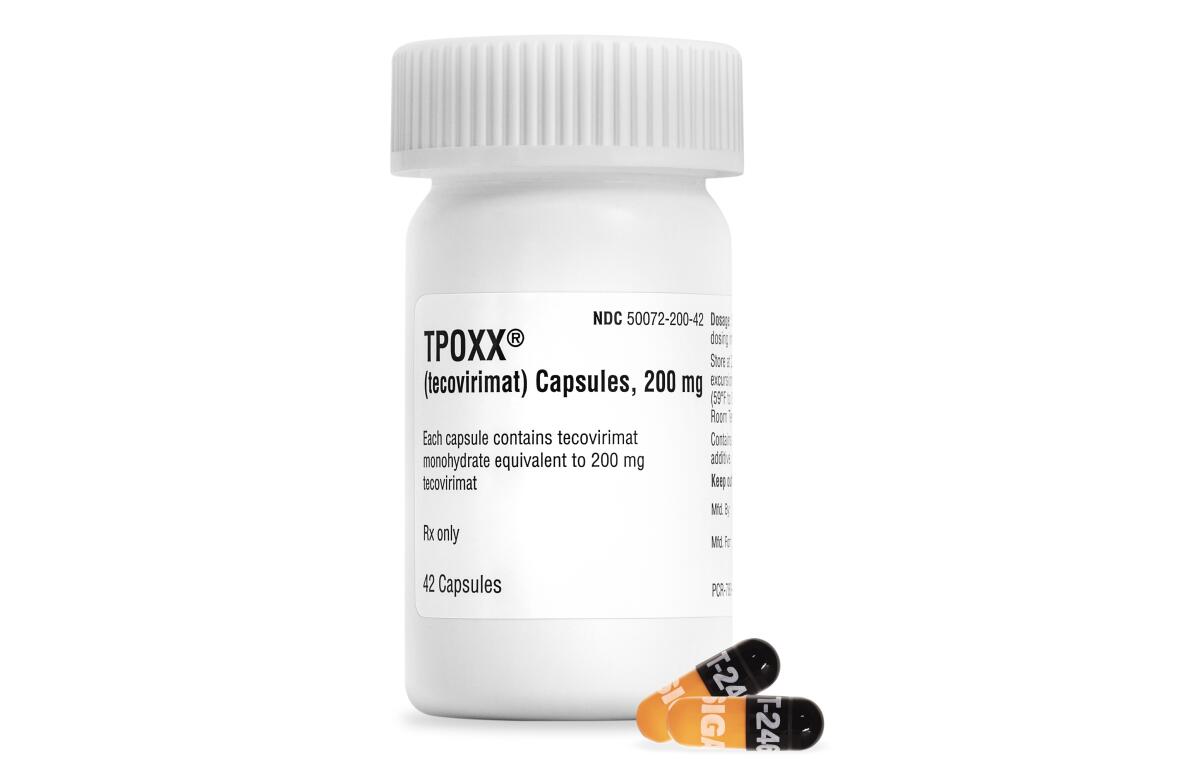
The Food and Drug Administration updated its guidance this week for Tpoxx, which has been prescribed to tens of thousands of MPX patients.
In an online update, FDA officials cautioned that a single molecular change to the virus “could have a large impact on the antiviral activity of Tpoxx.” Since viruses are constantly evolving to overcome obstacles to infection, including drugs, regulators stressed, doctors should be “judicious” in prescribing the medication.
Meanwhile, the Centers for Disease Control and Prevention on Thursday said Tpoxx should no longer be given to otherwise healthy adults who are not suffering severe symptoms.
“For most patients with healthy immune systems, supportive care and pain control may be enough,” agency officials said in a statement.
Editor's Note:
In light of widespread concerns that the name “monkeypox” is racist and stigmatizing, the World Health Organization has renamed the disease “mpox.”
The moves to scale back Tpoxx’s use follow weeks of criticism from HIV advocates and other patient groups who have urged the Biden administration to make the antiviral drug more widely available. Tpoxx is approved for the related smallpox virus, and its use against MPX is considered experimental and tightly controlled by federal officials.
Doctors wishing to prescribe the drug must submit an application to the CDC, documenting their patient’s need and agreeing to track their results and any side effects. Officials have shipped 37,000 courses of the drug to physicians.
Tpoxx works by targeting a single protein found on the MPX, smallpox and similar viruses. The FDA said this week that research in labs, animals and people suggests multiple ways in which the MPX virus could develop resistance to the therapy.
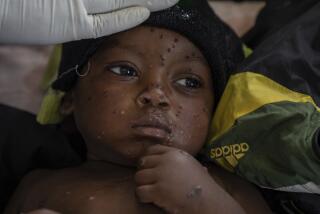
A Los Angeles County resident has died due to monkeypox, also known as MPX — the nation’s first confirmed fatality linked to the disease.
The update came as federal officials expressed cautious optimism about the trajectory of the outbreak, noting that new cases have fallen about 50% since their peak in August.
During a White House briefing Thursday, CDC Director Dr. Rochelle Walensky attributed the decline to vaccinations, educational outreach and individuals reducing behaviors linked to spread. The vast majority of U.S. cases have been in men who have sex with men, though officials emphasize that the virus can infect anyone.
Dr. Anthony Fauci, the nation’s top infectious disease official, noted that resistance is always a risk when using antiviral drugs.
“That’s why we’re uncomfortable when you only have a single drug,” Fauci said. He added that a recently launched study of Tpoxx supported by the National Institutes of Health will track signs of mutation that could lead to resistance. The study is expected to enroll more than 500 patients across 60 U.S. sites.
While MPX cases have started to slow in L.A. County and across California, officials are worried that disparities in cases and vaccine distribution are worsening.
Last month, the Biden administration invoked rare emergency powers to stretch the nation’s limited supply of MPX vaccines. And last week a separate declaration expedited the use of experimental tests for the virus.
But no changes were made to allow emergency use of Tpoxx, stoking complaints from groups representing gay and bisexual men.
The U.S. government’s national stockpile contains has more than 1.7 million courses of Tpoxx, originally manufactured to treat victims of a potential bioterrorism attack.
The FDA approved the medication in 2018 under its “animal rule,” which allows approval based on animal data when human testing is unethical or unfeasible. Smallpox was declared eradicated in 1980 by the World Health Organization, ruling out the possibility for human studies.
Experts around the world have pledged to change the disease’s name to something that doesn’t carry the weight of stigma. But tossing out the old term is easier than deciding on a new one.
Even though the drug was approved for smallpox, its effectiveness was measured in monkeys infected with MPX, considered a reasonable predictor of smallpox’s effect on humans. Animals receiving Tpoxx survived at higher rates than those on a placebo. But FDA officials have cautioned that results in animals must be confirmed in human testing.
“Without human trials, we don’t know if Tpoxx is beneficial for humans with monkeypox,” FDA Commissioner Dr. Robert Califf told Senate lawmakers at a hearing this week.
The CDC reported last week that 3.5% of patients tracked through its Tpoxx program reported side effects, mainly headache and nausea.
The agency has only gotten back about 200 forms from physicians documenting patients’ initial symptoms and results, accounting for less than 1% of the doses shipped since the start of the outbreak.
Stobbe reported from New York.