‘I don’t intend to experiment on my body’: Russia’s vaccine rollout draws mixed response
- Share via
MOSCOW — While excitement and enthusiasm greeted the U.S.-German Pfizer-BioNTech COVID-19 vaccine when it was rolled out, the Russian-made version has received a mixed response, with reports of empty Moscow clinics that offered the shot to healthcare workers and teachers — the first members of the public designated to receive it.
Kremlin officials and state-controlled media touted the Sputnik V vaccine as a major achievement after it was approved Aug. 11. But among Russians, hope that the shot would reverse the course of the coronavirus crisis has become mixed with wariness and skepticism, reflecting concerns about how it was rushed out while still in its late-stage testing.
Russia faced international criticism for approving a vaccine that has not completed advanced trials in tens of thousands of people, and experts both at home and abroad warned against its wider use until those studies are completed.
Despite those warnings, authorities started offering it to certain high-risk groups, such as front-line medical workers, within weeks of approval. Alexander Gintsburg, head of the Gamaleya Institute, which developed the vaccine, said last week that more than 150,000 Russians have gotten it.
One recipient was Dr. Alexander Zatsepin, an ICU specialist in Voronezh, a city 300 miles south of Moscow, who received the vaccine in October.
“We’ve been working with COVID-19 patients since March, and every day when we come home, we worry about infecting our family members. So when some kind of opportunity to protect them and myself appeared, I thought it should be used,” he said.
Touting a Russian vaccine is ‘huge gamble’ for Putin, but could carry rewards as well
But Zatsepin said he still takes precautions against infection because studies of the vaccine’s effectiveness aren’t over.
“There is no absolute confidence yet,” he said.
After Britain announced Dec. 2 that it had approved the Pfizer-BioNTech vaccine, Russian President Vladimir Putin told authorities to start a large-scale inoculation campaign, a sign of Moscow’s eagerness to be at the front of the vaccination race.
Russia approved its vaccine after it was tested on only a few dozen people, touting it as “the first in the world” to receive a go-ahead. Developers named it Sputnik V, a reference to the Soviet Union’s 1957 launch of the world’s first satellite during the Cold War.
The Russia-linked hacking group Cozy Bear is targeting academic and drug research institutions developing a vaccine, nations say.
More than just national pride is at stake. Russia has recorded more than 2.7 million coronavirus cases and over 49,000 deaths, and it wants to avoid another damaging lockdown of its economy.
On Dec. 2, Putin cited a target of more than 2 million doses in the coming days. Despite such a limited supply for a nation of 146 million, Moscow immediately widened who was eligible for it. Shots are free to everyone in medical or educational facilities, both state and private; social and municipal workers; retail and service workers, and those in the arts.
The EU’s medicines regulator said it has not received a request from the Russian vaccine-makers to consider licensing it for use in Europe, but some data have been shared with the World Health Organization. The U.N. agency does not typically approve vaccines itself but waits for regulatory agencies to weigh in first. Sputnik V is reportedly under consideration for use in a global effort led by the WHO to distribute COVID-19 vaccines to poorer countries.
Putin himself hasn’t received the shot yet. The 68-year-old Russian leader cited the age-based priority lists for vaccinations in Russia, telling the annual news conference Thursday that “vaccines have not yet reached people like me.”
“But I will definitely do it, as soon as it becomes possible,” Putin said.
It could be a while before U.S. efforts to develop coronavirus vaccines benefit the developing world. China and Russia are trying to fill the void.
The vaccine’s developers have said study data suggest it to be 91% effective, a conclusion based on 78 infections among nearly 23,000 participants. That’s far fewer cases than Western drugmakers have accumulated during final testing before analyzing their candidates’ efficacy, and important demographic and other details from the study have not been released.
Some experts say such efficacy rates inspire optimism, but public trust may be an issue.
“I don’t so much worry about Sputnik V being unsafe or less effective than we need it to be,” said Judy Twigg, a political science professor at Virginia Commonwealth University specializing in global health. “I worry about whether or not people are going to be willing to take it in Russia.”
A poll conducted in October by the Levada Center, Russia’s top independent pollster, showed that 59% of Russians were unwilling to get the shots even if offered for free.
Denis Volkov, sociologist and deputy director of the polling outfit, says respondents cited unfinished clinical trials, saying that the vaccine was “raw” and that they were suspicious of Russian claims to be the first country to have a vaccine while others were still working on theirs.
Some medical workers and teachers interviewed by the Associated Press expressed skepticism about the vaccine because it hasn’t been fully tested.
Dr. Yekaterina Kasyanova of Siberia’s Kemerovo region said she didn’t trust it enough to get the shot and has advised her mother, a teacher, not to get it either. “The vaccine is several months old. … Long-term side effects are not known, its effectiveness hasn’t been proven,” Kasyanova said.
Dzhamilya Kryazheva, a teacher in Krasnogorsk near Moscow, echoed that sentiment.
“I don’t intend to experiment on my body. I have three children,” she said.
Britain becomes the first country to approve a fully tested COVID-19 vaccine, but its show of national pride raises EU hackles.
For other healthcare workers, the choice to be vaccinated was easy.
“People are dying here every day. Every day, we carry out corpses. What’s there to think about?” said Dr. Marina Pecherkina, an infectious-disease specialist in the eastern city of Vladivostok. She got her shots in October because of her daily work with coronavirus patients.
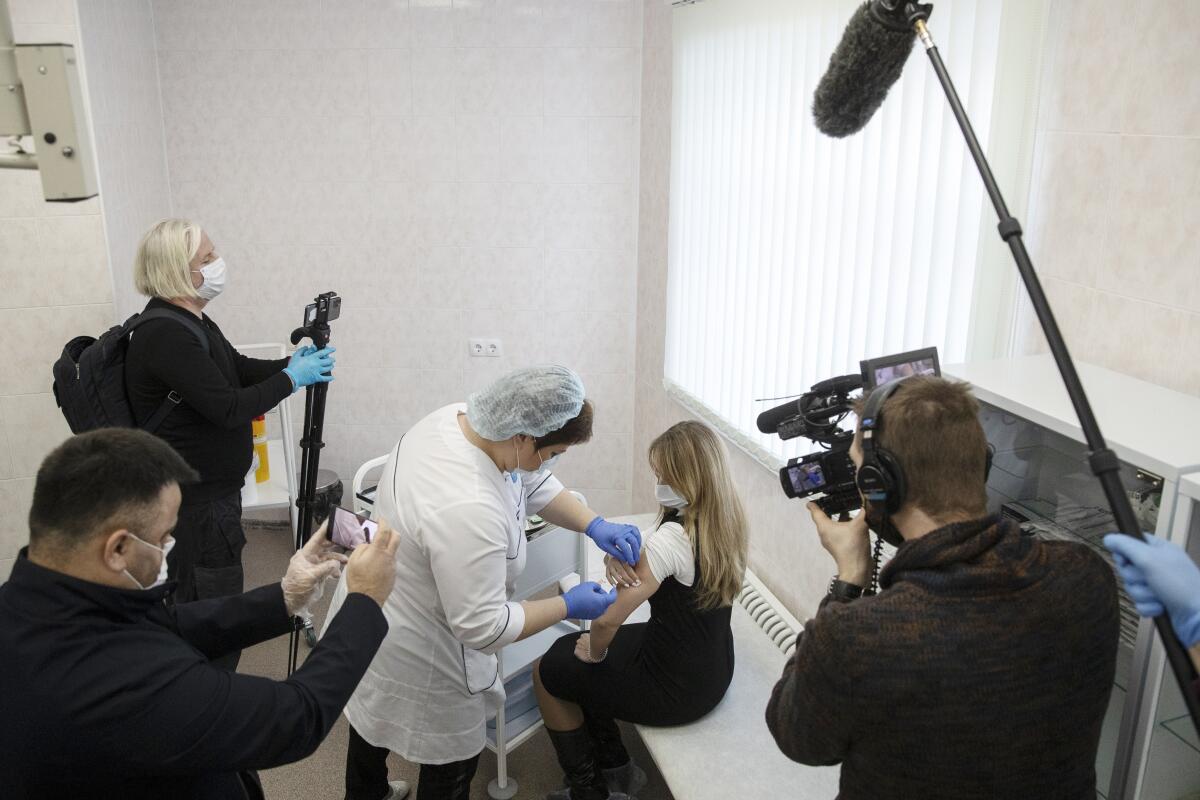
Moscow Mayor Sergei Sobyanin said about 15,000 people received the shots since vaccinations started Dec. 5. But some media reports on the first days of the Moscow campaign showed empty clinics and medical workers offering the shots to anyone who walked in. In some instances, this was because the vaccine must be stored at minus-0.4 degrees, and each vial contains five doses. Once defrosted, it must be administered within two hours or discarded.
The rollout outside Moscow and the surrounding region appeared to go much slower, with Health Minister Mikhail Murashko declaring that all regions started the vaccination Dec. 15.
U.S. officials say they’re actively negotiating for additional purchases of Pfizer’s coronavirus vaccine after passing up a chance to lock in a contract this summer because it was still unclear how well the shots would work
Media reports suggested there may be problems with scaling up the manufacture and distribution of Sputnik V. It uses two different adenovirus vectors for the two-shot regimen, which complicates production. In addition, the low-temperature storage required makes it harder to move across the vast country.
There also were confused signals about whether recipients should consume alcohol. Deputy Prime Minister Tatyana Golikova said those getting vaccinated should refrain from drinking three days before and after the shots.
Several medical workers in Siberia who received the vaccine later reported contracting the coronavirus, but health officials said not enough time had passed for them to develop the antibodies.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.