China gives conditional approval to first domestic COVID vaccine

- Share via
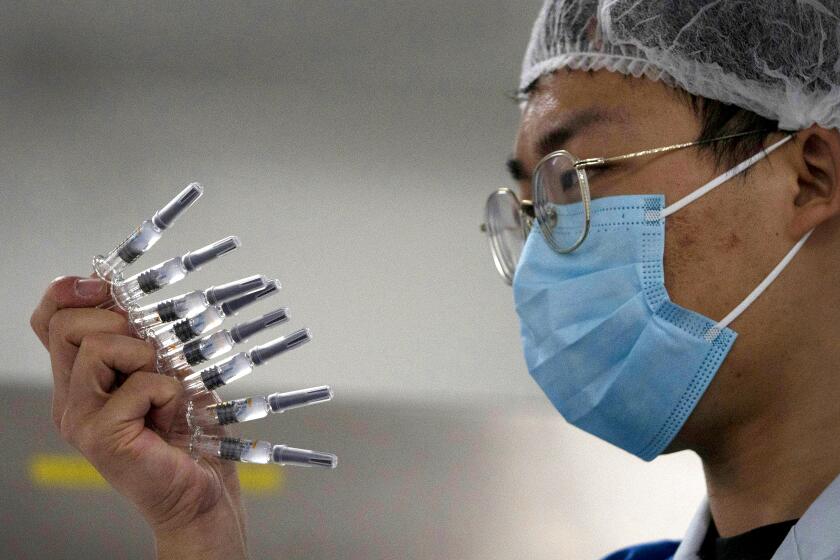
BEIJING — Chinese health regulators said Thursday that they have given conditional approval to a COVID-19 vaccine developed by state-owned Sinopharm.
The two-dose vaccine is the first approved for general use in China. The go-ahead comes as the country has begun to vaccinate 50 million people before the Lunar New Year holiday in February.
Conditional approval means that research is still ongoing. The company will be required to submit follow-up data as well as reports of any adverse effects after the vaccine is sold on the market, Chen Shifei, deputy commissioner of the National Medical Products Administration, said at a news conference.
The company “must continuously update the vaccine’s instructions, labels and report to the agency,” Shifei said.
The vaccine was developed by the Beijing Institute of Biological Products, a subsidiary of state-owned conglomerate Sinopharm. The company announced Wednesday that preliminary data from last-stage trials had shown it to be 79.3% effective.
China has already injected hundreds of thousands of people with COVID-19 vaccines. Vaccines that have not yet completed clinical trials.
It is an inactive vaccine, which means the virus was grown in a lab and then killed. The germ is then injected into the body to generate an immune response.
Final proof of its effectiveness will depend on publication of more data.
Sinopharm is one of at least five Chinese developers in a global race to create vaccines for the disease that has killed more than 1.8 million people worldwide.
In addition to the emergency vaccinations already underway, China plans to start vaccinating high-risk populations, including seniors and people with existing chronic illnesses. Officials did not say what percentage of the population they would vaccinate in China.
“This is different in every country, but the general thinking is that it has to reach 60% to protect the entire population,” said Zeng Yixin, vice minister of the National Health Commission.
Under emergency use, 4.5 million doses have already been given, including 3 million in the past two weeks, Zeng said.
Practically, the conditional approval means that the drug or product in question may be restricted for certain age groups, said Tao Lina, a former government immunologist.
Officials declined to name a particular price and gave conflicting statements about it. “It will certainly be in the limit of what people can afford,” said Zheng Zhongwei, another National Health Commission official.
Caught between the virus and the Trump-era crackdown on immigration, the elderly couple is trapped indefinitely in Southern California.
A minute later, Zeng, the other NHC official, stepped in to say that the vaccines “will definitely be free for the public.”
The vaccine is already under mass production, though officials did not answer questions about current production capacity.
Its approval could also mean hope for countries that may not have access to the Pfizer-BioNTech or Moderna-National Institutes of Health shots, which have stricter cold chain requirements. Sinopharm’s vaccine can be stored at 36 to 46 degrees Fahrenheit, a normal refrigeration temperature.
Sinopharm’s vaccine has already been approved in the United Arab Emirates and Bahrain, and is slated for use next in Morocco.
Other countries have also been buying doses of another Chinese vaccine candidate, made by Sinovac Biotech. Turkey received shipments this week of 3 million doses. Indonesia and Brazil have purchased Sinovac’s vaccines.
China is eager to distribute its vaccines globally, driven by a desire to repair the damage to its image by the pandemic that started a year ago in the central city of Wuhan.
President Xi Jinping has vowed to donate a Chinese-made vaccine as a public good to the world and China has joined COVAX, a global plan for equal distribution and access.
“We eagerly await Chinese vaccines to be included in COVAX’s vaccine bank soon and get WHO prequalification soon as well,” said Shen Bo, a Foreign Ministry official.
The vaccine standards were developed in “close cooperation” with the World Health Organization, officials said.
Meeting the WHO qualification could go some way toward assuring the rest of the world about the quality and efficacy of Chinese vaccines, which already face a reputation problem back home. It would also open the path for Chinese vaccines to be distributed in COVAX and potentially in countries that don’t have their own regulatory agencies.
“This is very exciting that there is another vaccine and one that can be distributed in locations that don’t have the cold chain,” said Ashley St. John, an immunologist at the Duke-NUS Medical School in Singapore. “But at the same time we have to temper the excitement. We have to understand the long term efficacy, effect on transmission and effect on severe disease.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.