Why are Germany, France and others halting use of AstraZeneca’s COVID-19 vaccine?
- Share via
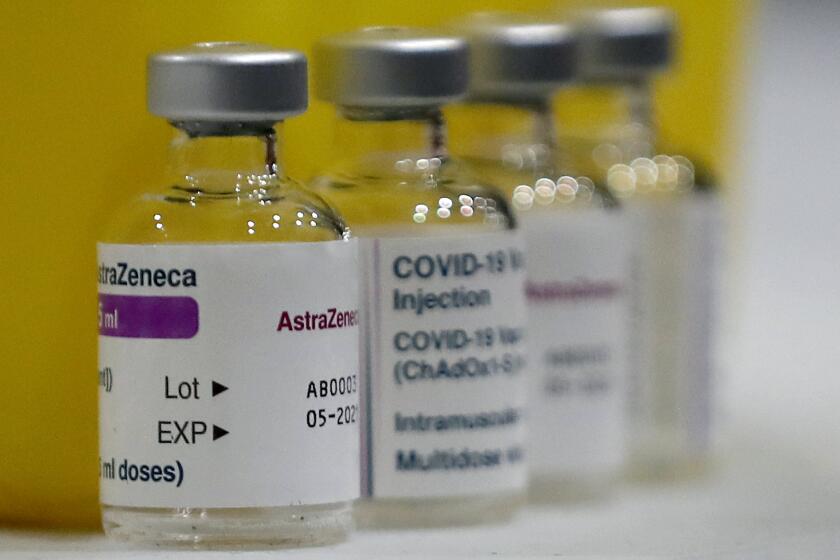
LONDON — In recent days, a handful of countries, including Germany, France, Denmark, Ireland and Thailand, have temporarily suspended their use of AstraZeneca’s COVID-19 vaccine after reports that some recipients developed blood clots, even though there is no evidence that the shot was responsible. The European Medicines Agency and the World Health Organization say that the data available do not suggest that the vaccine caused the clots and that people should continue to be immunized.
Here’s a look at we know — and what we don’t.
What happened?
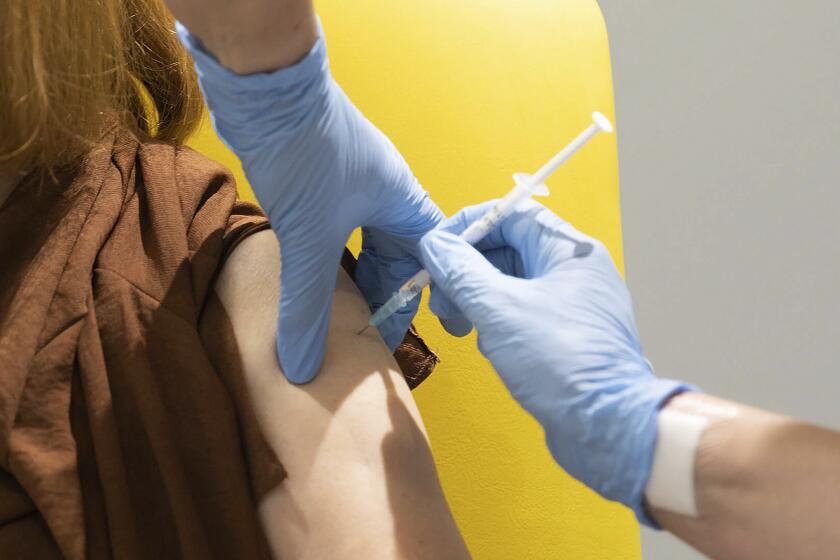
Denmark was the first country last week to halt use of the vaccine developed by AstraZeneca and Oxford University after reports of blood clots in some people, including one person who developed multiple clots and died 10 days after receiving at least one dose. Danish health authorities said the suspension would last for at least two weeks while the cases were investigated, although they noted that “at present, it cannot be concluded whether there is a link between the vaccine and the blood clots.”
Norway, Iceland, Bulgaria, Thailand and Congo soon followed suit. On Saturday, Norwegian authorities reported that four people under age 50 who had gotten the AstraZeneca vaccine had an unusually low number of blood platelets. That could lead to severe bleeding. On Sunday, Ireland and the Netherlands announced that they, too, were stopping their use of the AstraZeneca vaccine temporarily.
The U.K. has approved a second COVID-19 vaccine. AstraZeneca says it aims to supply millions of doses in the first quarter.
Authorities in the Netherlands — like those elsewhere — said their suspension of the AstraZeneca vaccine was strictly precautionary.
“We must always err on the side of caution, which is why it is sensible to press the pause button now as a precaution,” said Hugo de Jonge, the Dutch health minister.
Several other countries have continued using the vaccine.
In response to the suspensions, AstraZeneca said it had carefully reviewed the data on 17 million people who received doses across Europe. It said there was “no evidence of an increased risk” of blood clots in any age group or gender in any country.
Is there any proof the vaccine was responsible?
No. The European Medicines Agency says there is “no indication that vaccination has caused these conditions.” The EU regulator said the number of reports of blood clots in people who received the AstraZeneca vaccine was no higher than for those who hadn’t gotten the shot.
In Britain, where 11 million doses of the AstraZeneca vaccine have been administered — more than any other country — there have been reports of about 11 people who developed blood clots after getting a shot. None was proved to have been caused by the vaccine.
A new study may help answer a big question in the campaign to suppress the coronavirus outbreak.
Some doctors pointed out that since vaccination campaigns started by giving doses to the most vulnerable people, those now being immunized are more likely to already have health problems. Experts say that could make it difficult to determine whether a vaccine shot is responsible.
So why did they stop vaccination?
Any time vaccines are rolled out widely, scientists expect some serious health issues and deaths to be reported, simply because millions of people are receiving the shots and problems would be expected to occur randomly in a group so large. The vast majority of these end up not being connected to the vaccine, but because COVID-19 vaccines are still experimental, scientists must investigate every possibility that the shot could have some unforeseen side effects.
The shots are considered experimental because the vaccines were developed only in the last year, so there is no long-term data for any of them.
“People die every day, and we have more than 300 million people globally who have been immunized who will die of other causes,” said Dr. Mariangela Simao, an assistant director-general at WHO.
Mixing and matching doses of different COVID-19 vaccines was an idea that scientists dismissed out of hand. Now they’re taking it seriously.
Is this a concern with other COVID-19 vaccines?
The EMA is currently examining whether COVID-19 shots made by Pfizer, Moderna and AstraZeneca might be causing low levels of blood platelets in some patients, a condition that could lead to bruising and bleeding.
Has AstraZeneca run into other trouble?
The vaccine has been approved for use in adults in more than 50 countries and has been proved to be safe and effective in research done in Britain, Brazil and South Africa. But concerns have been raised about how the vaccine data have been released, and some European leaders, including French President Emmanuel Macron, have questioned the vaccine’s effectiveness.
Britain first authorized the vaccine based on partial results that suggested the shots were about 70% effective. But those results were clouded by a manufacturing mistake that led some participants to get just a half-dose in their first shot — an error that the researchers didn’t immediately acknowledge. When it recommended the vaccine be licensed, the EMA estimated the vaccine’s efficacy to be about 60%.
Scotland’s COVID-19 vaccination program has led to a sharp drop in hospitalizations, offering some real-world evidence of the shots’ impact.
The data on whether the vaccine protected older adults were also incomplete, leading some European countries to initially withhold the shot from older people.
In the U.S., the Food and Drug Administration suspended a study in 30,000 Americans for an unusual six weeks, as frustrated regulators sought information about some possible side effects reported in Britain.
“All the data we have seen about the AstraZeneca vaccine suggests it’s very safe and is saving people from dying of COVID,” said Paul Hunter, a professor of medicine at the University of East Anglia. “But this may be more of a perception problem because every time there is a vaccine issue, we hear the name ‘AstraZeneca’ soon after.”
So what are experts telling people to do?
The WHO and the EMA — as well as regulators in several countries — say that people should continue to be immunized and that the risk of getting vaccinated far outweighs any potential harm.
“The safety of the public will always come first,” said Britain’s drug regulator. “People should still go and get their COVID-19 vaccine when asked to do so.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.