Supreme Court temporarily keeps FDA abortion pill rules in place
- Share via
WASHINGTON — The Supreme Court said Friday it was temporarily keeping in place federal rules for use of an abortion drug while it takes time to more fully consider the issues raised in a court challenge.
In an order signed by Justice Samuel A. Alito Jr., the court asked both sides to weigh in by Tuesday over whether lower court rulings restricting the Food and Drug Administration’s approval of the drug, mifepristone, should be allowed to take effect while the case works its way through federal courts. The order suggests the court will decide that issue by late Wednesday.
The justices are being asked at this point only to determine what parts of an April 7 ruling by U.S. District Judge Matthew Kacsmaryk in Texas, as modified by an appellate ruling Wednesday, can be in force while the case continues.
The Biden administration and New York-based Danco Laboratories, the maker of the pill, asked the justices to intervene.
In the order signed by Alito, the court put a five-day pause on the fast-moving case so the justices can decide whether lower court rulings restricting the FDA’s approval of mifepristone should be allowed to take effect in the short term.
The order expires late Wednesday, suggesting the court will decide that issue by then.
Rigorous research tracking the mental health of women who’ve had abortions doesn’t support the claim that mifepristone is psychologically damaging.
The court finds itself immersed in a new fight involving abortion less than a year after conservative justices reversed Roe vs. Wade and allowed more than a dozen states to effectively ban abortion outright.
A lawyer for the antiabortion doctors and medical organizations suing over mifepristone said the court’s action Friday was “standard operating procedure” and urged the justices to allow the appeals-court-ordered changes to take effect by the middle of next week.
The type of order issued by the court Friday, an administrative stay, ordinarily is not an indication of what the justices will do going forward. It was signed by Alito because he handles emergency filings from Texas. Alito also is the author of last year’s opinion overturning Roe vs. Wade.
The Justice Department and Danco both warned of “regulatory chaos” and harm to women if the high court doesn’t block the lower-court rulings that had the effect of tightening FDA rules under which mifepristone can be prescribed and dispensed.
The new limits would have taken effect Saturday if the court hadn’t acted.
“This application concerns unprecedented lower court orders countermanding FDA’s scientific judgment and unleashing regulatory chaos by suspending the existing FDA-approved conditions of use for mifepristone,” Solicitor General Elizabeth Prelogar, the Biden administration’s top Supreme Court lawyer, wrote Friday, less than two days after the appellate ruling.
Gov. Gavin Newsom announced a stockpile of 2 million abortion pills known as misoprostol after a Texas judge ruled against using a mifepristone, another medication to terminate pregnancies.
The Biden administration and Danco now want a more lasting order that would keep the current rules in place as long as the legal fight over mifepristone continues. As a fallback, they asked the court to take up the issue, hear arguments and decide by early summer a legal challenge to mifepristone that antiabortion doctors and medical organizations filed last year.
The court rarely acts so quickly to grant full review of cases before at least one appeals court has thoroughly examined the legal issues involved.
A ruling from the 5th U.S. Circuit Court of Appeals late Wednesday would prevent the pill, used in the most common abortion method, from being mailed or prescribed without an in-person visit to a doctor. It also would withdraw the FDA’s approval of mifepristone for use beyond the seventh week of pregnancy. The FDA says it’s safe through 10 weeks.
Still, the appeals court did not entirely withdraw FDA approval of mifepristone while the fight over it continues. The 5th circuit narrowed the April 7 ruling by Kacsmaryk, whose far-reaching and virtually unprecedented order would have blocked FDA approval of the pill. He gave the administration a week to appeal.
“To the government’s knowledge, this is the first time any court has abrogated FDA’s conditions on a drug’s approval based on a disagreement with the agency’s judgment about safety — much less done so after those conditions have been in effect for years,” Prelogar wrote.
Erin Hawley, a lawyer for the challengers, said in a statement that the FDA has put politics ahead of health concerns in its actions on medication abortion.
“The 5th Circuit rightly required the agency to prioritize women’s health by restoring critical safeguards, and we’ll urge the Supreme Court to keep that accountability in place,” said Hawley, senior counsel with Alliance Defending Freedom, a conservative legal group that also argued to overturn Roe vs. Wade.
The Justice Department is appealing a Texas court ruling that would halt approval of the most commonly used method of abortion in the United States.
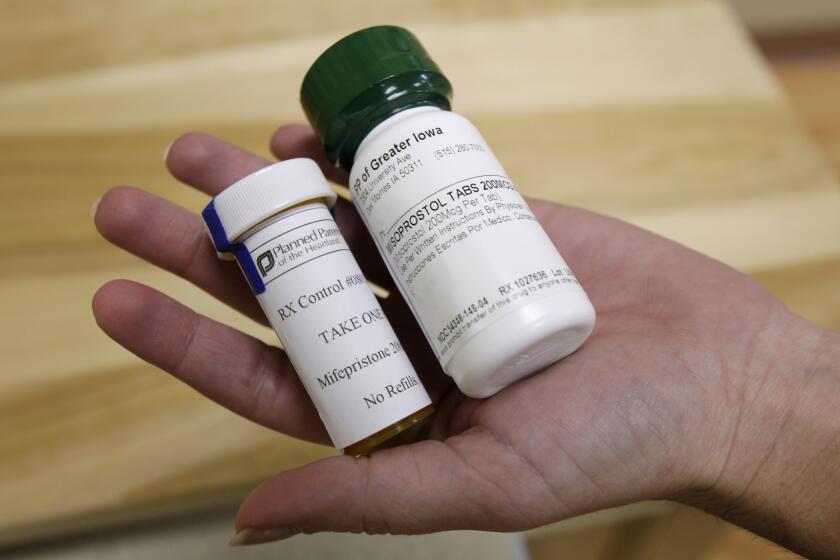
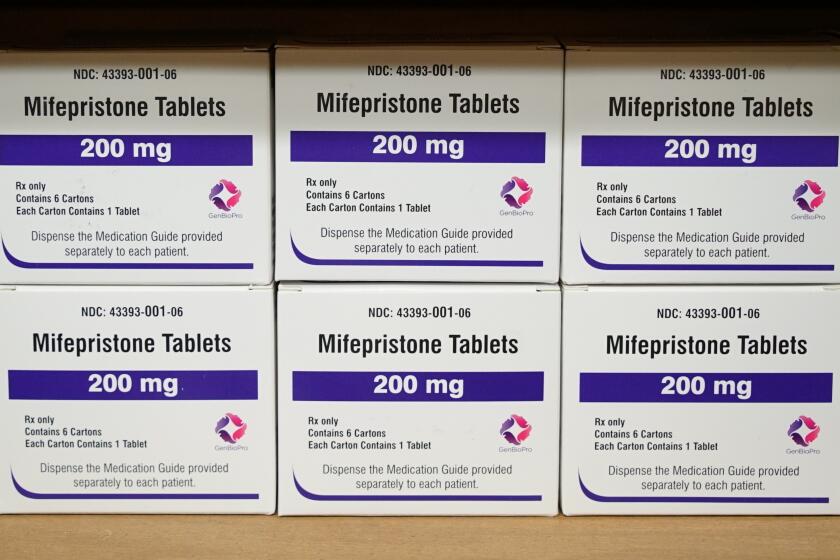
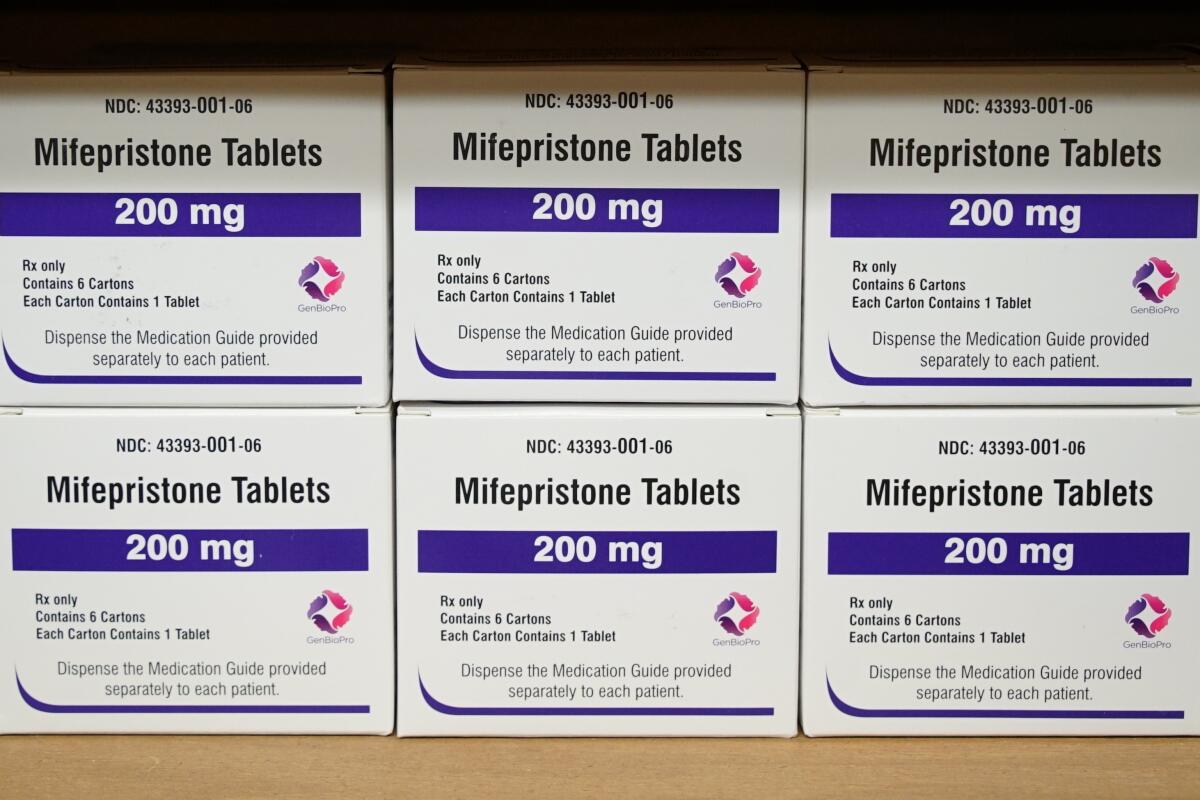
Mifepristone was approved by the FDA more than two decades ago and is used in combination with a second drug, misoprostol, for abortions.
Adding to the uncertainty, a separate federal judge in Washington on Thursday clarified his own order from last week to make clear that the FDA is not to do anything that might block mifepristone’s availability in 17 Democrat-led states suing to keep it on the market.
It’s unclear how the FDA can comply with court orders in both cases, a situation that Prelogar described Friday as untenable.
Use of medication abortion jumped significantly after the 2016 rule expansion, according to data gathered by the Guttmacher Institute, a research group that supports abortion rights. In 2017, medication abortion accounted for 39% of abortions but by 2020 had increased to become the most common method, accounting for 53% of all abortions.
Experts have said the use of medication abortion has increased since the court overturned Roe.
When the drug was initially approved, the FDA limited its use to up to seven weeks of pregnancy. It also required three in-person office visits: the first to administer mifepristone, the next to administer the second drug, misoprostol, and the third to address any complications. It also required a doctor’s supervision and a reporting system for any serious consequences of the drug.
If the appeals court’s action stands, those would again be the terms under which mifepristone could be dispensed for now. At the core of the Texas lawsuit is the allegation that the FDA’s initial approval of mifepristone was flawed because the agency did not adequately review safety risks.
The GOP-dominated Florida Legislature on Thursday approved a ban on abortions after six weeks of pregnancy, a proposal signed into law by Gov. Ron DeSantis later in the day.
Mifepristone has been used by millions of women over the last 23 years. While less drastic than completely overturning the drug’s approval, the ruling still represents a stark challenge to the FDA’s authority overseeing how prescription drugs are used in the United States. The panel overturned multiple decisions made by FDA regulators after years of scientific review.
Common side effects with mifepristone include cramping, bleeding, nausea, headache and diarrhea. In rare cases, women can experience excess bleeding that requires surgery to stop.
Still, in loosening restrictions on mifepristone, FDA regulators cited “exceedingly low rates of serious adverse events.” More than 5.6 million women in the U.S. had used the drug as of June 2022, according to the FDA. In that period, the agency received 4,200 reports of complications in women, or less than one-tenth of 1% of women who took the drug.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.