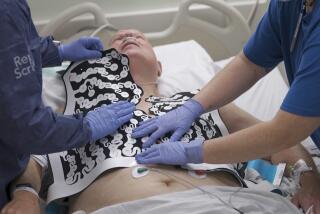
Cardiac Science Inc.: The Irvine company said...
- Share via
Cardiac Science Inc.: The Irvine company said Thursday that it has received approval from the Food and Drug Administration to start the second phase of human clinical trials of its non-surgical defibrillator device for high-risk heart patients. The trials are expected to begin in the next quarter at the Arizona Heart Institute in Phoenix. Phase I testing was completed at UC Irvine Medical Center, Montefiore Medical Center in New York, and the Arizona Heart Institute in Phoenix.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.