Mark Berman, pusher of unproven stem cell therapies, dies while awaiting verdict in FDA lawsuit
- Share via
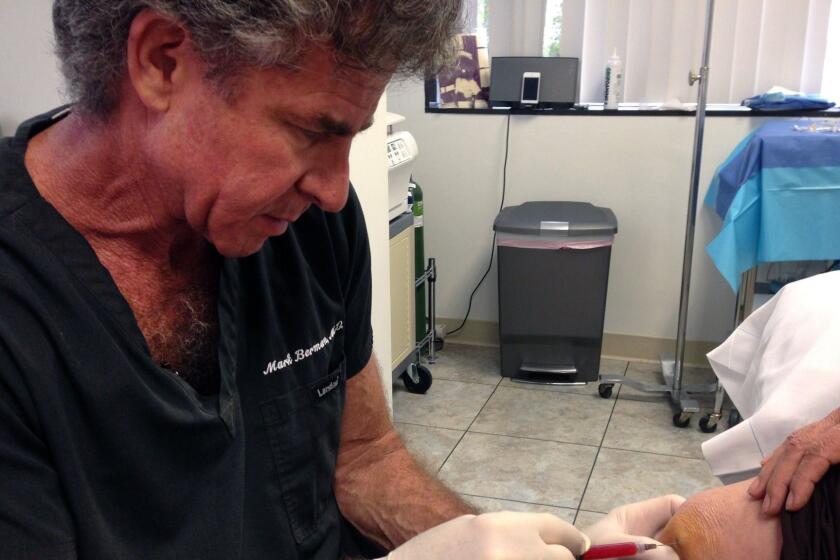
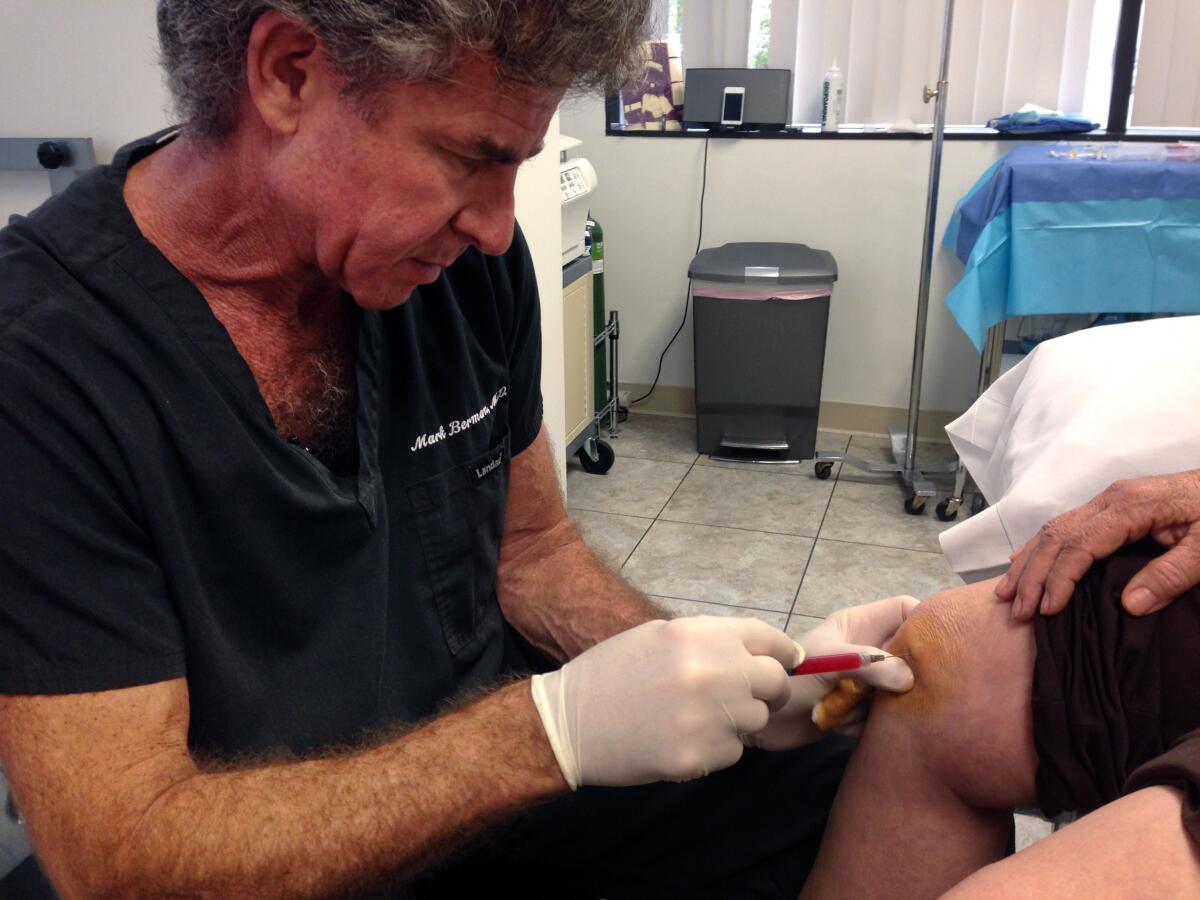
For years, California cosmetic surgeon Mark Berman was a leader of that corner of the healthcare world pushing unproven and unapproved stem cell treatments for a host of medical conditions.
Berman aired his claims for what he called “magic cells” in a book, video appearances and through a network of affiliated clinics around the country. Those claims caught the attention of the Food and Drug Administration, which has been trying to stamp out clinics claiming that stem cell injections can treat diseases and conditions such as Alzheimer’s, Parkinson’s, autism and even— most recently — COVID-19.
I cannot condone exploitation of desperate people who are led to believe that they will be cured or even helped by a clinic or a pill or any purported therapy that is not based in science.
— Jeanne Loring, Scripps Research Institute
A 2018 lawsuit the FDA filed against Berman, his professional partner Elliot Lander and their two stem cell businesses is awaiting a verdict from U.S. District Judge Jesus Bernal in Riverside. Whichever way it goes, Bernal’s ruling would set the stage for the next phase of the FDA’s campaign against stem cell clinics — either endorsing its position that the injection procedure amounts to administering illegal drugs or erecting an obstacle to enforcement of its rules.
Berman died April 19, according to his office. His death hasn’t been publicly announced. An email sent last week to a patient and signed by an office manager at Cell Surgical Network, which Berman co-founded with urologist Elliot Lander in 2012, stated that he died after being hospitalized in early January.
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.
Lander told me by email, “The family will release all pertinent information in an obituary which has not been completed and published yet.” Berman’s son, Sean, confirmed that an obit is being written.
Berman’s death provides us with an opportunity to review the FDA’s campaign against clinics purveying bogus stem cell treatments.
The agency’s targets are clinics that rely on a method similar to the one promoted by Berman and his colleagues at clinics branded as the California Stem Cell Treatment Center in Rancho Mirage and Beverly Hills and at scores of clinics affiliated with the Cell Surgical Network. The treatment centers were co-founded by Berman and Lander in 2010.
The method starts with extracting fat from a customer via liposuction. The fat is then refined to isolate what is known as the “stromal vascular fraction,” or SVF, which Berman asserted is rich in stem cells. The SVF is then reinjected into the customer.
Hiltzik: After the FDA delayed enforcement against stem cell clinics, unlicensed operators poured in
The FDA gave stem cell clinics three years to comply with its rules. The number of illegal clinics exploded.
Customers of more than 1,000 clinics around the country, some of which are affiliated with the Cell Surgical Network, have been charged as much as several thousand dollars for such procedures, which are seldom, if ever, covered by health insurance.
In a 2015 book titled “The Stem Cell Revolution,” Berman and Lander asserted that their colleagues in the network were treating “a wide range of conditions” such as Alzheimer’s, autism, cerebral palsy, multiple sclerosis, muscular dystrophy, Parkinson’s, stroke and traumatic brain injury.
More recently, Berman, Lander and other stem cell promoters have been implying that stem cells can be used to treat COVID-19.
The FDA’s position on these claims is crystal clear. The agency observes that there is no scientifically validated evidence for those treatment claims. The agency has warned prospective customers to stay away from the claims’ promoters.
“Don’t believe the hype,” the agency says on its website. “Some unscrupulous providers offer stem cell products that are both unapproved and unproven,” as well as “potentially dangerous.”
The FDA says it has not approved stem cell treatments for any conditions other than rare blood system diseases, for which it has approved treatments using blood-forming stem cells derived from umbilical cord blood. The agency has specifically warned against claims that stem cells can be used to treat autism, macular degeneration, blindness, chronic pain, fatigue; neurological disorders such as multiple sclerosis, Alzheimer’s and Parkinson’s; or cardiovascular or pulmonary diseases.
The agency also has warned against claims, which are also unproven, that stem cells can be used for “the treatment or prevention of COVID-19, acute respiratory distress syndrome (ARDS), or any other complication related to COVID-19.”
In practical terms, however, the FDA’s campaign has not been going well. In 2017, the FDA closed a loophole that allowed the stem cell clinics to continue pitching unproven, ineffective and potentially hazardous stem cell therapies directly to consumers. The stem cells extracted from fat, the FDA ruled, were drugs under the law, meaning that the clinics would need to be licensed and subjected to inspection.
But the agency unwisely gave clinics up to three years to comply with its new regulations. Then it extended the deadline by six months because of the pandemic, so its period of regulatory “forbearance” expired May 31, 2021.
Hiltzik: Don’t be taken in by stem cell firms offering unsubstantiated therapies for COVID-19
Clinics with unproven stem cell treatments are already targeting COVID-19 fears.
By then, a torrent of shady operations had poured into the field — so many that the task of protecting the public from them may exceed the FDA’s capabilities. According to a study by Leigh Turner of UC Irvine, by midyear 2021 there were 1,480 businesses operating 2,754 clinics nationwide.
“That hardly seems like progress,” Turner told me last year. “The problem the FDA faces is four times larger than what existed in 2016. The FDA only has so many employees and so many inspectors. They don’t really have enough inspectors to send them to 1,480 businesses.”
Before his death at age 69, Berman and his associates reportedly had been studying whether stem cells could be used to treat COVID. In testimony during the federal trial, Berman stated that Cell Surgical Network was working with a Florida stem cell company, American Cell Technology, “to treat COVID-19 patients” in accordance with a treatment protocol the network developed, as an FDA post-trial brief filed in June describe his statements from the witness box.
Hiltzik: U.S. judge rejects FDA bid to shut down stem cell clinics, dealing blow to regulators
A U.S. judge throws a wrench into the FDA’s campaign against stem cell clinics.
Sean Berman, Mark Berman’s son, who says he is a consultant to ACT, told me that as far as he is aware, no patients have been treated for COVID according to the protocol the FDA mentioned.
Lander seemed to indicate, however, that a study of some type had taken place. “No patients were ever charged any fees in our Covid-19 study,” he told me by email.
Details about Berman’s death have not been made public.
A person who answered the phone at California Stem Cell Treatment Center told me that Berman died of “complications from Covid.”
Lander, however, told me: “Any person who represented themselves to be an agent of California Stem Cell over the phone was neither knowledgeable enough, nor authorized, to discuss Dr. Berman’s medical history.”
One patient says Berman’s staff canceled a pre-op procedure in January, stating that Berman had COVID. The patient, who does not want to be identified, says Berman’s office kept offering to reschedule the appointment, but ultimately the procedure did not take place.
The FDA has been looking at Berman’s operations at least since 2017. In two inspection reports in July 2017, the agency listed numerous “objectionable conditions” at the California Stem Cell Treatment Center facilities in Rancho Mirage and Beverly Hills that it asserted were in violation of regulatory standards .
In what may be the harshest enforcement order yet against a clinic pitching unproven and potentially harmful stem cell “treatments,” a federal judge has ordered a Florida-based stem cell firm to cease offering the treatments or face thousands of dollars in fines.
The reports criticized sterilization procedures, training and record-keeping at the locations. At Rancho Mirage, at least four “serious adverse events” after stem cell procedures had not been investigated by the clinic or reported to the FDA as required, the agency said.
They included a patient’s retinal detachment after the SVF was injected into the patient’s eyes, hospitalization of another patient for “confusion and headache,” and two cases of infection.
Berman and Lander wrote in their book that they had found “extraordinary safety and no serious adverse effects related to SVF deployment.”
The FDA’s most determined attack on Berman came in the lawsuit filed in Riverside federal court in May 2018, aimed essentially at forcing him and his facilities to cease treating patients with the fat extractions. The lawsuit was largely identical to one the agency filed the same day against U.S. Stem Cell, a Florida clinic operator, in Miami federal court.
Both sets of plaintiffs mounted similar defenses — chiefly that they were merely treating patients with their own tissues, so their products fell outside the FDA definition of a drug and thus were exempt from the agency’s jurisdiction. The FDA disagreed. The extracted tissues were so thoroughly manipulated before reinjection, the agency argued, that they were properly defined as a drug and didn’t qualify for the exemption.
The two lawsuits have followed divergent paths. In Miami, District Judge Ursula Ungaro agreed with the FDA in a major victory for the agency’s campaign against stem cell clinics.
Ungaro held in June 2019 that the SVF was indeed a drug and therefore used illegally. She further observed that U.S. Stem Cell’s procedures “involve a higher risk of communicable disease and other safety concerns and are therefore subject to FDA regulation.” Her decision siding with the agency via summary judgment — that is, without trial — effectively shut down U.S. Stem Cell’s clinic.
Ungaro’s ruling was upheld in June by the U.S. 11th Circuit Court of Appeals in Atlanta.
In Riverside, however, Bernal denied the FDA’s motion for summary judgment. Instead, he held a seven-day trial in May 2021. Post-trial briefs by both sides were submitted in August, but Bernal has yet to issue a verdict. There are no indications of when he might rule.
Berman’s death won’t require Bernal to suspend his deliberations, as there are still three other defendants — Lander, California Stem Cell Treatment Center and Cell Surgical Network.
The FDA, in its post-trial brief in June, properly raised the alarm that the defendants might be aiming their pitch at victims of COVID-19, despite the lack of evidence that stem cells are effective in treating the disease. Why should that be a surprise? The field of stem cell clinics has been feeding on the desperation of sick people virtually since its inception.
“Has a doctor ever told you ‘there’s nothing we can do?’” Berman and Lander wrote in their book. “We sincerely hope you won’t accept that as the final answer.” The FDA’s response has been, in effect: Beware, because they don’t have the answer either.
More to Read
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.