WHO says Trump’s anti-coronavirus drug is best left to clinical tests
- Share via
GENEVA — The World Health Organization, which has come under repeated fire from President Trump, says that the old malaria drug he’s taking to try to ward off the coronavirus is better left to controlled clinical trials for now.
Dr. Michael Ryan, the WHO’s emergencies chief, said that hydroxychloroquine — which Trump said Monday that he‘s taking — is just one of many possible therapies being tested internationally to see if they are effective against the coronavirus.
Ryan’s comments late Wednesday suggested that the WHO remains unbowed by Trump’s repeated criticism over its response to the coronavirus pandemic, including most recently his threat to end all funding for the U.N. health agency from the U.S., its biggest donor, if it doesn’t reform.
Ryan nonetheless emphasized that countries can make their own choices.
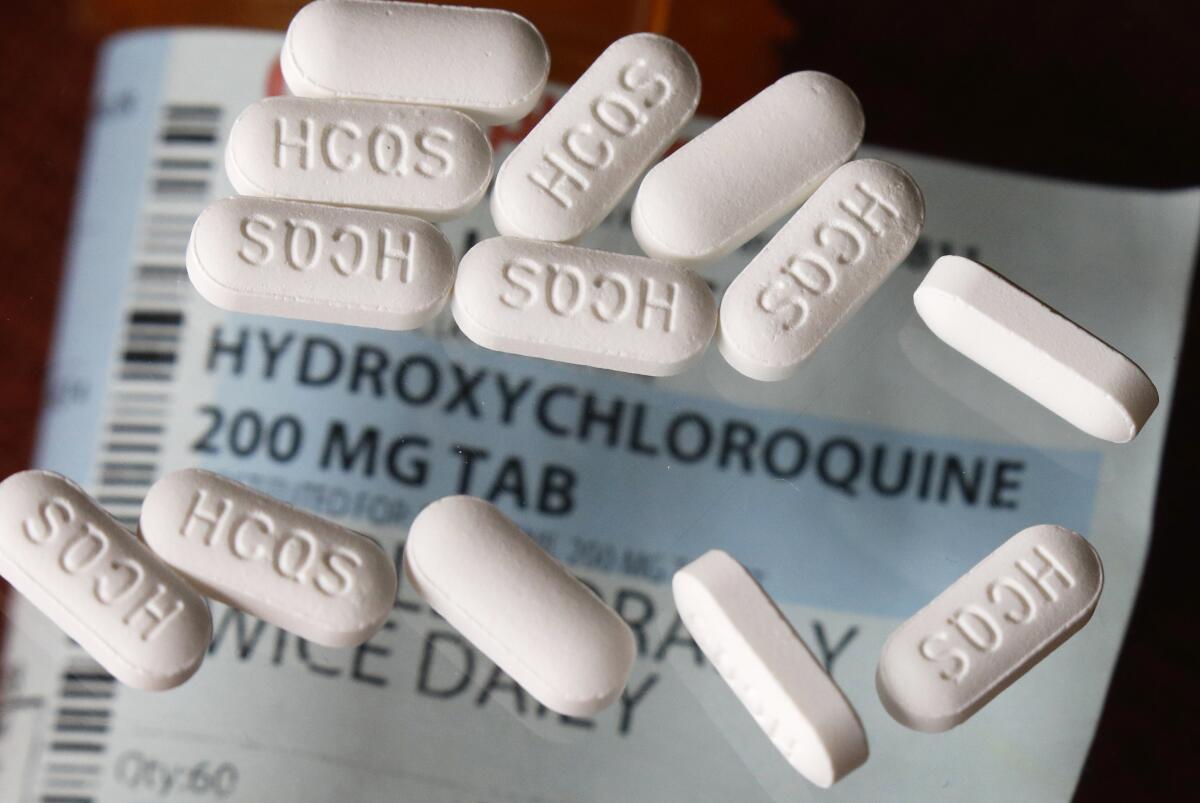
“Every sovereign nation, particularly those with effective regulatory authorities, is in a position to advise its own citizens regarding the use of any drug,” he said. “I would point out, however, that at this stage [neither] hydroxychloroquine nor chloroquine have been as yet found to be effective in the treatment of COVID-19 or in the prophylaxis against coming down with the disease. In fact, the opposite — in that warnings have been issued by many authorities regarding the potential side effects of the drug.”
The FDA has issued a warning contradicting President Trump’s advice on drugs to treat COVID-19 after patients experienced heart issues.
Based on laboratory, animal and clinical studies, the WHO is overseeing what it calls “Solidarity Trials” involving a number of countries on four possible treatments for COVID-19: remdesivir, which was previously tested as an Ebola treatment; the HIV treatment lopinavir and ritonavir; multiple sclerosis treatment interferon beta-1a; and related drugs chloroquine and hydroxychloroquine, which have been used to treat illnesses including malaria and rheumatoid arthritis.
“As WHO, we would advise that for COVID-19 these drugs be reserved for use within such trials,” Ryan said.
Trump’s own administration has warned that hydroxychloroquine can have deadly side effects, and both the European Medicines Agency and the U.S. Food and Drug Administration warned health professionals last month that the drug should not be used to treat COVID-19 outside hospital or research settings because of numerous serious side effects that in some cases can be fatal.
Drug company AstraZeneca has booked orders for a potential coronavirus vaccine and won $1 billion in funding from the U.S. government.
Trump has repeatedly criticized the WHO for its early response to the coronavirus outbreak and what he considers its excessive praise of China, where the outbreak began, at a time when his administration’s own response in the U.S. has come under scrutiny.
Trump has already ordered a pause in U.S. funding for the WHO, which totaled nearly $900 million in 2018-19, according to information on the agency’s website. That represented about one-fifth of its total $4.4-billion budget for those years.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.