With OK from experts, some states resume use of J&J vaccine
- Share via
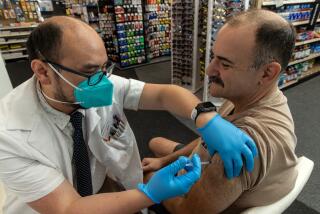
NEW YORK — With a green light from federal authorities, several states resumed use of the one-shot Johnson & Johnson COVID-19 vaccine on Saturday.
The states moved quickly after U.S. health officials said Friday evening they were lifting an 11-day pause on vaccinations using the J&J vaccine. During the pause, scientific advisors decided the vaccine’s benefits outweighed a rare risk of causing blood clots.
Among the states ordering or recommending a resumption were California, Indiana, Arizona, Colorado, Louisiana, Michigan, Missouri, Nevada, New York, Tennessee and Virginia.
Gov. Gavin Newsom and work crews stood on the freshly paved and marked roadway to celebrate the reopening of the main artery to and from Big Sur.
In Los Angeles County, the nation’s most populous county, public health officials told vaccine providers they could resume administering J&J doses on Saturday, as long as they provided an updated fact sheet to recipients.
Dr. Paul Simon, chief science officer for the county’s Department of Public Health, said the county has been working on developing additional materials to explain the clotting issue that prompted the pause.
Those will “include what we think is really important information about what to look for — the signs and symptoms if you were to have this, again, very rare reaction,” he said. “And we are going to underscore that this is a very rare reaction.”
Across the country, Gov. Andrew Cuomo said in a statement Saturday morning, “The state of New York will resume administration of this vaccine at all of our state-run sites effective immediately.”
“The vaccine is the weapon that will win the war against COVID and allow everyone to resume normalcy, and we have three proven vaccines at our disposal,” Cuomo said, urging New York residents to take whichever one is available to them first.
“The sooner we all get vaccinated, the sooner we can put the long COVID nightmare behind us once and for all,” he said.
The Indiana Department of Health announced resumption of a free COVID-19 mass vaccination clinic Saturday at the Indianapolis Motor Speedway.
“I can’t think of a better way to welcome the month of May in Indiana than getting your vaccine this week at the Yard of Bricks,” said Dr. Chris Weaver, chief clinical officer for Indiana University Health, which is partnering with the state in running the speedway clinic.
Virginia health officials also told providers to immediately resume their use of the J&J vaccine.
“This extra scrutiny should instill confidence in the system that is in place to guarantee COVID-19 vaccine safety,” said Dr. Danny Avula, the state’s vaccine coordinator. “As with any vaccine, we encourage individuals to educate themselves on any potential side effects and to weigh that against the possibility of hospitalization or death from COVID-19.”
Avula received the J&J vaccine himself on April 1.
Missouri officials made a similar announcement, saying providers with J&J vaccine in stock can immediately begin administering it and that shipments from the federal government will resume next week.
Just over 105,000 doses of J&J had been administered in Missouri before the pause.
In Michigan, where local health departments have a key role in vaccination decision making, the state’s chief medical executive, Dr. Joneigh Khaldun, recommended resuming use of the J&J vaccine.
The federal government uncovered 15 vaccine recipients who developed a highly unusual kind of blood clot out of nearly 8 million people given the J&J shot. All were women, most under age 50. Three died, and seven remain hospitalized.
But ultimately, federal health officials decided that J&J’s one-and-done vaccine is critical to fight the pandemic — and that the small clot risk could be handled with warnings to help younger women decide if they should use that shot or an alternative, and with the development of treatments for those who do suffer complications.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.