Britain becomes first country to approve Pfizer’s COVID-19 vaccine
- Share via
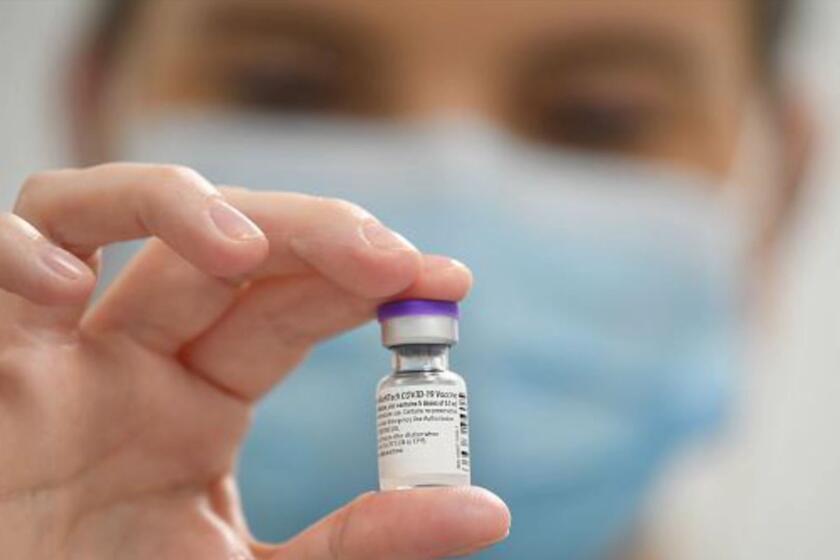
FRANKFURT, Germany — Britain has become the first country to approve a COVID-19 vaccine following large-scale clinical trials, after its regulator authorized the shot developed by Pfizer and Germany’s BioNTech for emergency use.
Pfizer and BioNTech said doses of the vaccine, which uses novel mRNA technology, would be delivered to the U.K. in the “coming days” after the country’s Medicines and Healthcare products Regulatory Agency, or MHRA, announced the approval Wednesday morning.
“The vaccine will begin to be made available across the UK from next week,” Prime Minister Boris Johnson tweeted.
When people talk about COVID-19 vaccines, they can sound like they’re speaking a foreign language. Don’t worry! Here’s your guide to vaccine vocabulary.
The vaccine appears to be 95% effective in preventing the disease, according to data released last month from the companies’ Phase 3 trial, which involved more than 43,000 people.
A British government spokesperson said: “The vaccine will be made available across the U.K. from next week. The NHS [National Health Service] has decades of experience in delivering large-scale vaccination programs and will begin putting their extensive preparations into action to provide care and support to all those eligible for vaccination.”
The inoculation is still under review by U.S. and EU regulators. A decision from the U.S. Food and Drug Administration is expected in mid-December, but the Amsterdam-based European Medicines Agency has said it is unlikely to approve the shot until the end of the month.
Federal regulators will consider requests in December by Pfizer and Moderna for emergency use authorization for their coronavirus vaccines.
China and Russia have approved COVID-19 vaccines for early or limited use, but both countries authorized the shots without waiting for the results of Phase 3 trials, provoking criticism from some experts who cautioned that the rushed process was risky.
Britain was the first country to reach an agreement with BioNTech and Pfizer for the supply of vaccines, ordering 30 million doses in July. It has since signed a deal for an additional 10 million doses. About 10% of the Britain’s total order is expected to be delivered before the end of the year.
“We believe that the rollout of the vaccination program in the U.K. will reduce the number of people in the high-risk population being hospitalized,” said Ugur Sahin, co-founder and chief executive of BioNTech.
Much of the U.K.’s supply will be produced at Pfizer’s facilities in Puurs, Belgium. There will also be some production at BioNTech’s sites in Germany.
Lots more needs to be known about the Pfizer vaccine before COVID-19 is beaten.
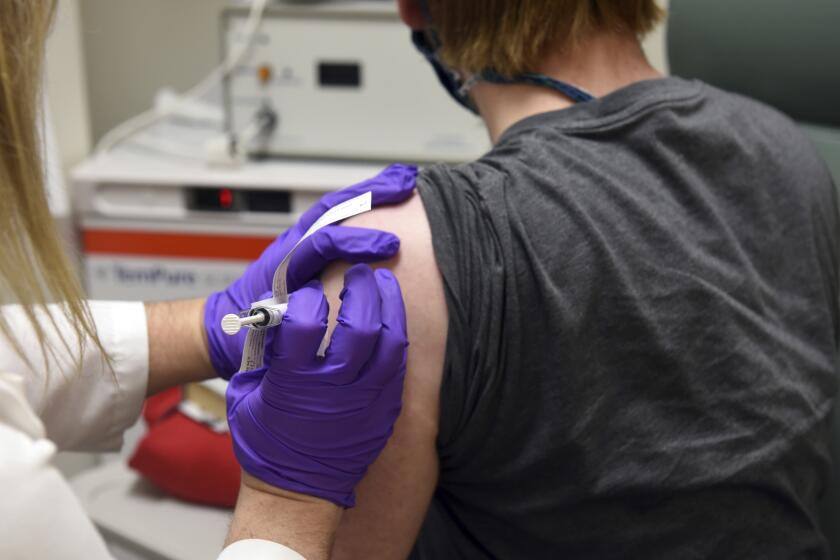
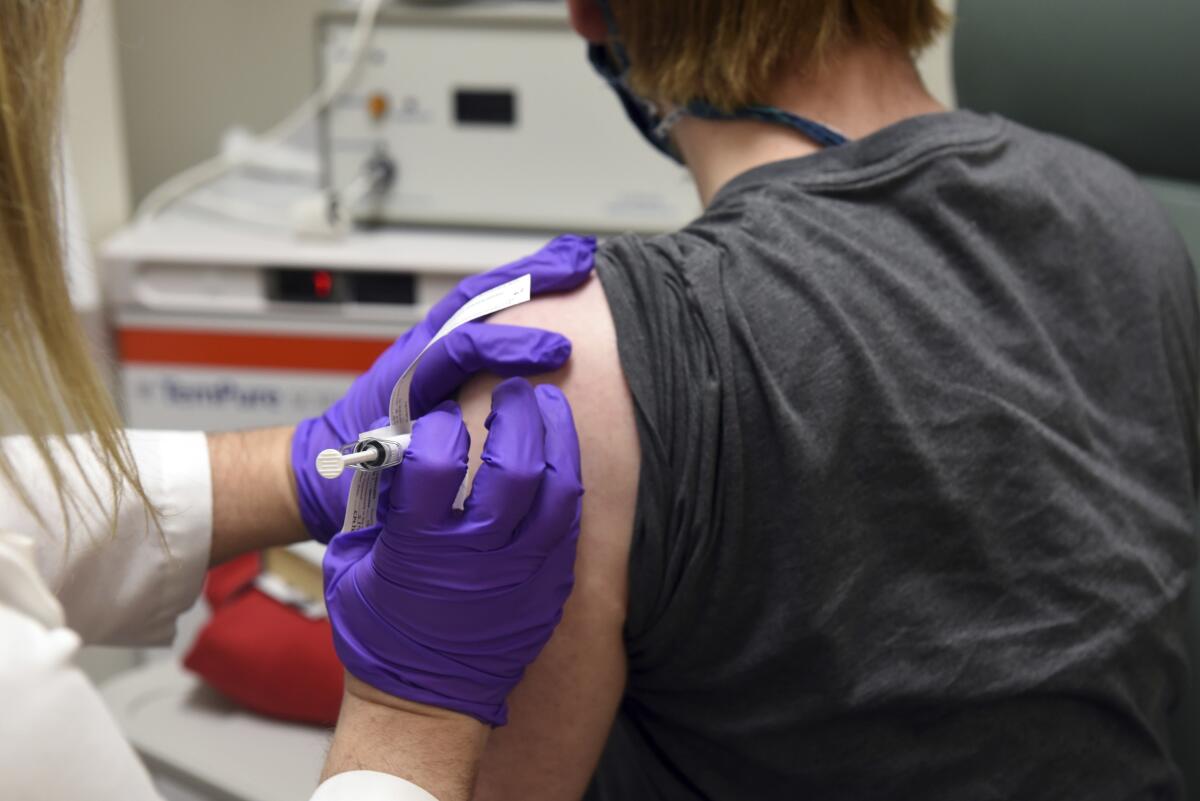
Under provisional plans released by the British government last month, the vaccine — which requires two shots, roughly a month apart — will first be made available to nursing home residents and staff before being administered to those over 80 and to front-line healthcare workers.
“I’m confident now, with the news today, that from spring — from Easter onwards — things are going to be better,” Health Secretary Matt Hancock told the BBC. “We’re going to have a summer next year that everybody can enjoy.”
Britain is due to leave the European Union’s regulatory framework when the Brexit transition period ends Dec. 31. It was able to approve the vaccine earlier than the rest of Europe by using a long-standing regulatory provision that allows it to diverge from the EU’s drug regulator in the case of urgent public need.
The MHRA is also examining data on vaccines developed by Moderna and the partnership between Oxford University and AstraZeneca. In total, the British government has secured 357 million doses of seven separate COVID-19 vaccines.
© The Financial Times Ltd. 2020. All rights reserved. FT and Financial Times are trademarks of the Financial Times Ltd. Not to be redistributed, copied or modified in any way.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.