Moderna asks regulators to OK its COVID-19 shot for children under 6
- Share via
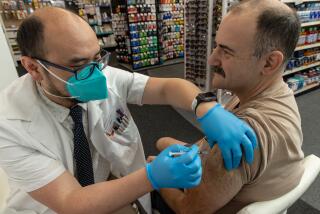
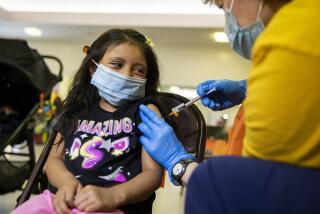
Moderna asked U.S. regulators Thursday to authorize its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially making shots available for millions of kids by summer.
The company submitted data to the Food and Drug Administration that it hopes will prove two low-dose shots can protect babies, toddlers and preschoolers, albeit not as effectively during the Omicron surge as earlier in the pandemic.
Frustrated families have waited impatiently for a chance to protect the nation’s littlest kids as all around them people shed masks and other public health precautions — even though highly contagious coronavirus mutants continue to spread.
“There is an important unmet medical need here with these youngest kids,” Dr. Paul Burton, Moderna’s chief medical officer, told the Associated Press. Two kid-size shots “will safely protect them. I think it is likely that over time they will need additional doses. But we’re working on that.”
Now, only children ages 5 or older can be vaccinated in the U.S., using rival Pfizer’s vaccine, leaving 18 million younger kids unprotected.
Moderna’s vaccine isn’t the only one in the race. Pfizer is soon expected to announce if three of its even smaller-dose shots work for the littlest kids, months after the disappointing discovery that two doses weren’t quite strong enough.
Hospitalizations have yet to follow the path of rising coronavirus cases in California, and in fact are at near-record lows.
Whether it’s one company’s shots or both, FDA vaccine chief Dr. Peter Marks said the agency would “move quickly without sacrificing our standards” in deciding if doses for very young children are safe and effective.
While questions are swirling about what’s taking so long, Marks pointedly told lawmakers this week that the FDA can’t evaluate a product until a manufacturer completes its application. FDA will publicly debate the evidence with its scientific advisors before making a decision, and Marks said multiple meetings would be set to cover several expected applications.
“It’s critically important that we have the proper evaluation so that parents will have trust in any vaccines that we authorize,” Marks told a Senate committee.
If the FDA clears the vaccinations for the youngest of children, the Centers for Disease Control and Prevention would next have to recommend who needs them — all children in that age group or just those at higher risk from COVID-19.
Taiwan had been living mostly free of COVID-19 until this month
Many parents are desperate for whichever vaccine gets to the scientific finish line first.
“We’ve been kind of left behind as everybody else moves on,” said Meagan Dunphy-Daly, a Duke University marine biologist whose 6-year-old daughter is vaccinated but whose 3-year-old and 18-month-old sons are part of Pfizer’s trial.
The family continues to wear masks and take other precautions until it’s clear if the boys received real vaccine or dummy shots. If it turns out they weren’t protected in the Pfizer study and Moderna’s shots are cleared first, Dunphy-Daly said she’d seek them for her sons.
“I will feel such a sense of relief when I know my boys are vaccinated and that the risk of them getting a serious infection is so low,” she said.
Falling coronavirus infection rates across Latin America have led to the easing of rules on mass gatherings, travel and mask-wearing.
Some parents have even urged the government to let families choose shots before all the evidence is in.
“This strain of COVID feels almost impossible to dodge,” Dana Walker, a mother of an 8-month-old, tearfully told a CDC meeting last week. “Cut red tape and allow parents to protect their kids.”
The FDA will face some complex questions.
In a study of kids aged 6 months through 5 years, two Moderna shots — each a quarter of the regular dose — triggered high levels of virus-fighting antibodies, the same amount proven to protect young adults, Burton said. There were no serious side effects, and the shots triggered fewer fevers than other routine vaccinations.
But the vaccine proved about 40% to 50% effective at preventing symptomatic COVID-19 during the trial. Burton blamed the Omicron variant’s ability to partially evade vaccine immunity, noting that adults without booster shots showed similarly less ability to fight milder Omicron infections. While no children became severely ill during the study, Burton said high antibody levels were a proxy for protection against more serious illness — and the company would test a child booster dose.
Another issue: So far in the U.S., Moderna’s vaccine is restricted to adults. Other countries have expanded the shot to kids as young as 6. But months ago the FDA cited concern about a rare side effect, heart inflammation, in teenage boys, and it hasn’t ruled on Moderna’s earlier pediatric applications.
Burton said the FDA may consider Moderna’s vaccine for children of all ages — but also might open it first to the youngest kids, who have no other option. He said safety data from millions of older children given Moderna vaccinations abroad should help reassure parents.
While COVID-19 generally isn’t as dangerous in youngsters as adults, some do become severely ill or even die. About 475 children younger than 5 have died from COVID-19 in the U.S. since the pandemic’s start, according to the CDC, and child hospitalizations soared at Omicron’s peak.
Yet it’s not clear how many parents intend to vaccinate their very young kids. Fewer than a third of children aged 5 to 11 have had two vaccinations, and 58% of those aged 12 to 17.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.