Stanford begins testing Pfizer COVID-19 vaccine on children as young as 2
- Share via
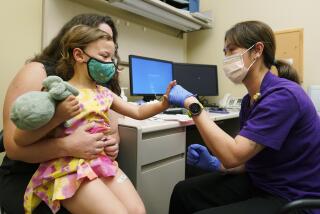
As statewide eligibility for the COVID-19 vaccine expands to residents 16 and older, researchers at Stanford Medicine have set their sights on an even younger group: children ages 2 to 5.
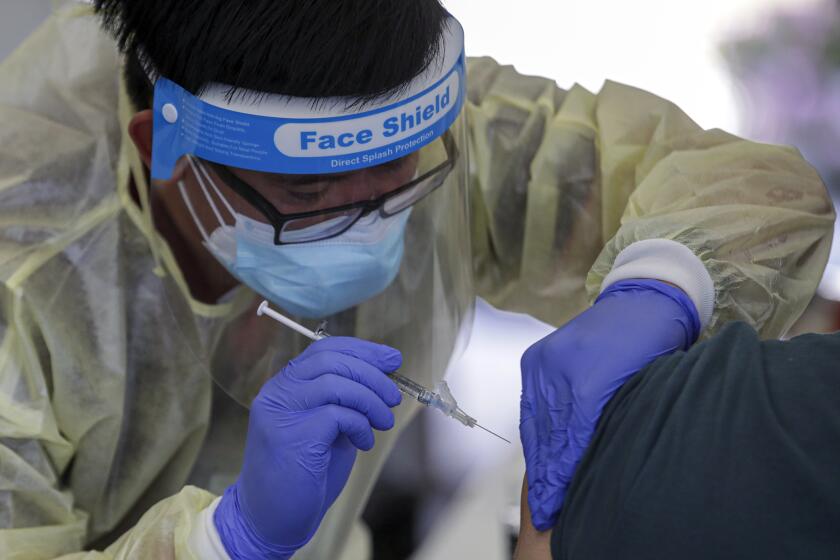
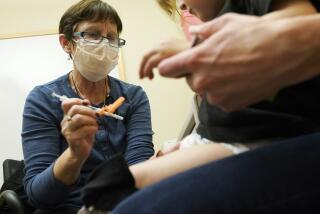
The medical school Wednesday began administering doses to children in the tender age group as part of a larger, three-phase trial of Pfizer-BioNech’s COVID-19 vaccine that will ultimately include children ages 6 months to 12 years.
“We want to protect children just as we want to protect adults from this disease,” said Dr. Yvonne Maldonado, the pediatric infectious diseases expert leading the trial at Stanford. “The goal is to have a pediatric vaccine available for all age groups from 6 months of age to adulthood.”
About 76 million Americans have been fully vaccinated against the novel coronavirus, according to the Centers for Disease Control and Prevention, but children remain unprotected even as they head back to school. Currently, the Pfizer vaccine is available to people age 16 and older, while the Moderna vaccine is available to people 18 and older.
U.S. officials urged that use of the one-dose Johnson & Johnson vaccine be put on hold while federal investigators look into reports of serious yet rare blood clots. That vaccine was previously available to people 18 and older.
The first phase of the Pfizer study is geared toward finding a safe dosage for preschool-age children, while the second and third phases will include a study of efficacy, Maldonado said.
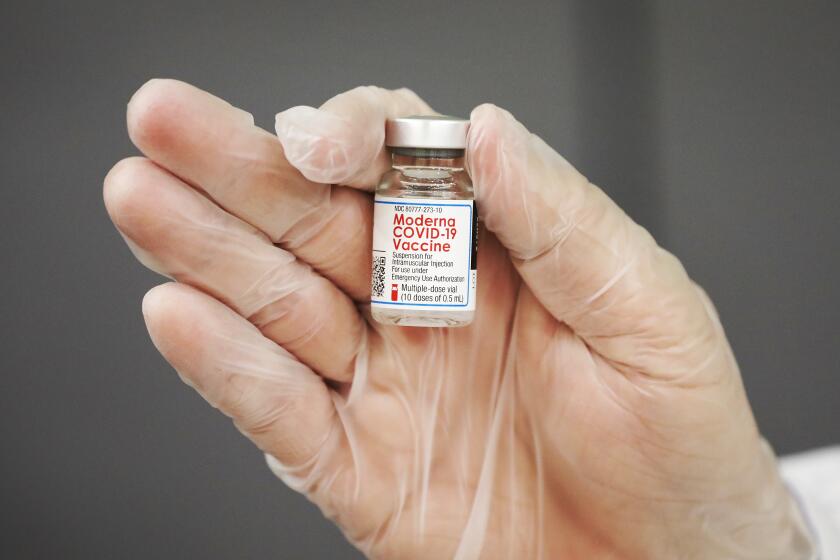
The trial will start by testing a 10-microgram dose of the vaccine, then progress to doses of 20 and 30 micrograms if it safe to do so, according to Pfizer. For comparison, people 16 and older receive two 30-microgram doses 21 days apart.
A past coronavirus infection may eliminate the need for a second dose of COVID-19 vaccine, a growing body of evidence suggests.
“The goal is really to find the right dose for little kids and make sure they tolerate the dose, don’t develop high fevers or any adverse events from these vaccines,” Maldonado said. “Once we get a good dose that seems to work well, we will then move into phases two and three.”
The study will include 144 volunteers in phase one, and up to 4,500 in phases two and three, according to Pfizer. Stanford is one of five nationwide sites participating in the trial, and the only one on the West Coast. People interested in signing their children up for the trial can enroll here.
The trial arrives as Moderna conducts its own study to determine whether its vaccine is safe and effective in children as young as 6 months.
Moderna has begun testing its COVID-19 vaccine in children as part of a study to determine if it is safe and effective in kids as young as 6 months.
Early vaccine development efforts were centered around adults, who are significantly more likely to develop a serious case of COVID-19. But young people, initially believed to be all but impervious to the virus, have also been affected: More than 3.5 million people under age 20 have tested positive nationwide, and nearly 300 have died of COVID-19, according to an American Academy of Pediatrics report from April 8.
In California, nearly 13% of confirmed cases have been people under 17, according to state data.
A COVID-related illness called multisystem inflammatory syndrome (MIS-C) has also affected nearly 170 children in the state, 26% of whom were under 5. There is no reliable way to predict an immune response that leads to MIS-C, but it is possible that COVID-19 vaccines will provide some degree of protection, according to the AAP.
In addition to protecting young people, vaccinations are important for slowing the spread of the virus within the larger community, Maldonado said.
“Children make up 20% or more of the U.S. population, and we want to be able to provide that herd immunity for all of our population to really reduce transmission of the virus,” she said.
Two COVID-19 vaccines are likely to be protective against a rapidly spreading variant of the SARS-Cov-2 virus that arose in California, a study finds.
In March, Dr. Anthony Fauci, the U.S. government’s top infectious-disease expert, said the country won’t achieve herd immunity until children are vaccinated.
“We don’t really know what the magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape,” he told the Senate Health, Education, Labor and Pensions Committee. “We ultimately would like to get — and have to get — children into that mix.”
Last month, Pfizer announced that their COVID-19 vaccine was 100% effective in a study of participants ages 12 to 15. The company has requested amendments to the U.S. Food and Drug Administration’s emergency use authorization to include adolescents in that age group.
That request is awaiting the FDA’s review, the company said.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.