Battle over ‘biosimilars’
- Share via
One of the most promising frontiers in healthcare is biologic medicines — complex substances derived from living cells that can help fight chronic diseases and cancers. To encourage investment in biologics, Congress in 2010 gave drug companies what amounts to a 12-year monopoly on the substances they developed. Now, supporters of biologics are pushing lawmakers in Sacramento and other state capitals to put new hurdles in the way of knock-off compounds, called “biosimilars.”
The debate over biosimilars is grounded in doubts about their safety; none have yet been approved for use in the United States. Proponents of a bill by California Sen. Jerry Hill (D-San Mateo) to regulate the dispensing of biosimilars include critically ill patients who fear that the new medicines won’t match the biologics they rely on, as well as doctors who prescribe and study biologics. Supporters also include the biotechnology companies whose expensive biologics account for about a quarter of U.S. pharmaceutical revenue — a share that’s expected to reach $100 billion in 2015.
On the other side stand the generic drug companies that want to make biosimilars. They see measures like Hill’s as a thinly veiled attempt by leading biologic manufacturers Amgen and Genentech to hold off competition. They’ve won support from U.S. Food and Drug Administration Commissioner Margaret Hamburg, who warned against reducing the public’s confidence in biosimilars. She recently predicted that competition from biosimilars would “spur innovation, improve consumer choice and drive down medical costs,” just as the generic versions of brand-name pills have done.
Clearly, patient safety has to be policymakers’ top priority. But state lawmakers shouldn’t substitute their own judgment for the FDA’s scientific analysis. And they need to balance the very real needs of the patients taking biologics against the public’s interest in affordable healthcare. That’s why they should be wary of impeding the arrival of biosimilars that the FDA deems interchangeable with their biologic counterparts.
Drug makers have been extracting some naturally occurring biologics, such as insulin and vaccines, from human and animal tissues for decades. More recently, however, biotechnology companies came up with ways to genetically engineer cells to synthesize therapeutic compounds. Because biologics’ molecules are larger and more intricate than those of the typical medicine, there’s a greater risk that a patient’s immune system will respond in an unhealthy way. Even small alterations in the manufacturing process can change a biologic enough to produce a different immune response.
Experts say there is no way at this point for generic drug makers to create a perfect copy of a biologic, although it could conceivably be possible in the future. That’s why the industry uses the term “biosimilar” instead of “generic biologic.” Nevertheless, European regulators have allowed biosimilars to be sold there since 2006. And in the 2010 healthcare law, Congress opened the door to biosimilars in the U.S., provided that they passed muster with the FDA.
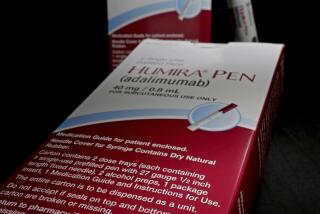
The FDA has yet to issue final guidelines for would-be biosimilar manufacturers, and no company has applied yet for approval of such a compound. Nevertheless, Amgen and Genentech have been lobbying across the country for tougher dispensing restrictions on biosimilars than those on generic pills. These include requirements that a pharmacist obtain the permission of the prescribing physician or the patient before substituting a biosimilar for a biologic.
Hill’s proposal, SB 598, stops well short of that. Pharmacists would be free to replace a biologic with a biosimilar deemed “interchangeable” by the FDA provided that they informed the patient and kept a record of the substitution for three years, the same as with generic drugs. The one extra step is that they would be required to notify the prescribing physician within five days of the switch.
To patients and physicians worried about the differences between a biosimilar and its biologic counterpart, this kind of notification is only prudent. Should a previously undetected and harmful immune response emerge after a patient starts taking a biosimilar, they say, it’s crucial that the doctor know the medication was different from what was prescribed.
This argument assumes that the FDA can’t or won’t comply with federal law, which allows the “interchangeable” designation only for biosimilars that are no less safe and effective than the original biologic, and can be substituted with no detrimental effect. To surmount that extremely high bar, biosimilar manufacturers will have to conduct clinical trials and supply evidence that there’s no
difference in how patients respond. The FDA already has to make similar judgments when the makers of approved biologics seek
permission to change the manufacturing process.
Rather than jumping ahead of the FDA, state lawmakers should wait to see the guidance the agency gives for biosimilars, particularly its requirements for monitoring for adverse effects after the substances go on the market. In the meantime, they should consider how to improve the dissemination of information about adverse reactions to all prescription medicines, not just biosimilars. Treating physicians too often lack vital information about the medications a patient is taking, and valuable insights about drug responses and interactions don’t necessarily get shared.
Biologics can cost $10,000 or more a month per patient, and as their usage expands, those costs will put increasing pressure on the rest of the healthcare system. Because they are cheaper to develop, biosimilars could cut those expenses significantly. Ultimately, the more slowly healthcare costs grow, the more people who’ll be able to afford the care they need. The state ought not to impede the adoption of biosimilars before the FDA shows how well it can enforce the interchangeability standard.
More to Read
A cure for the common opinion
Get thought-provoking perspectives with our weekly newsletter.
You may occasionally receive promotional content from the Los Angeles Times.