Coronavirus Today: Outdoor dining in jeopardy
- Share via
Good evening. I’m Karen Kaplan, and it’s Tuesday, Feb. 14. Happy Valentine’s Day! Here’s the latest on what’s happening with the coronavirus in California and beyond.
The pandemic has changed many things about the way we live, and in my opinion, some of those changes ought to be permanent. A prime example: the proliferation of outdoor dining options.
Parking spaces are convenient, but they’re nowhere near as lovely as the wooden planters, rustic benches and inviting cafe tables that have turned asphalt into gathering spots.
In Los Angeles, these urban oases were made possible by L.A. Al Fresco, a pandemic program that simplified the permitting process for restaurants looking to increase their capacity by converting sidewalks, streets and parking lots into outdoor seating areas. Instead of spending hundreds or thousands of dollars to file an application that might take months for the city to act on, L.A. Al Fresco enabled more than 2,500 businesses to get permits immediately by applying online for free.
When the program was introduced in the spring of 2020, it was seen as a way to help restaurants stay in business and keep their workers employed. It also created a safer environment for patrons.
One thing it was never meant to be was permanent.
“The original intent behind L.A. Al Fresco was to provide restaurant operators the ability to temporarily keep their doors open during the height of the pandemic, as a result of waivers granted through the emergency orders,” said Yeghig Keshishian, the chief external affairs officer for L.A. City Planning. “Now that those emergency orders are being lifted, the city must codify this program to preserve the original intent of L.A. Al Fresco.”
As a result, restaurants in Los Angeles that would like to maintain their outdoor seating will have to apply for new permits, according to the proposed ordinance. Unlike in 2020, these permit applications will be neither quick nor free. They won’t even be cheap.
Consider the dilemma facing the owners of Found Oyster in East Hollywood. An L.A. Al Fresco permit allowed the eatery to serve 20 diners in a parklet outside the front door. With that extra capacity, Found Oyster was able to stay open during the most challenging times of the pandemic. The owners would like to keep it to serve patrons who are still uncomfortable eating indoors with strangers.
But it’ll cost them. Holly Fox, a member of the restaurant’s ownership group, enumerated the various costs to my colleague Jenn Harris.
“If we have to build a new deck at Found Oyster, we’ll probably be spending $35,000 to $40,000 with furniture, plus any of the city fees and time,” she said. “Right now, a conditional use permit process by itself, I’m spending, with expedited fees, $10,000 to $12,000 to apply, another $15,000 to effectuate, and that doesn’t include the cost of consultants to get it done. That’s upward of $45,000 to $60,000, just for the conditional use permit approval, and that excludes building and safety fees, other permit fees and any construction costs.”
Christy Vega is facing a similar dilemma at Casa Vega, her restaurant in Sherman Oaks. She spent hundreds of thousands of dollars turning part of her parking lot into elegant outdoor seating that she thought would be permanent. But the terms of the proposed ordinance say her outdoor dining area can take up only five parking spaces. She can apply for a conditional use permit to maintain her patio, but the application will be expensive and take about a year to process.

Tyler Wells isn’t willing to take that gamble to retain the 60-seat patio he and his wife, Ashley, built for $30,000 at All Time, their restaurant in Los Feliz. Wells would need several new permits just to keep things the way they are now, and he has already plunked down $7,000 for a sidewalk permit at another property.
If the ordinance passes in its current form, All Time would lose half its outdoor seats. That loss of capacity would force the couple to restructure the business plan, which would definitely involve cutting staff, he said.
“The pandemic Al Fresco permit was the most thoughtful working program this city has ever offered to restaurants, and I can’t wrap my mind around it,” he said. “It feels very mean-spirited to take away things that have been a godsend.”
From the city’s point of view, it’s not taking things away so much as restoring rules about parking and hours of operation that had been set aside to help businesses cope with the pandemic.
“These rules did not go away with the emergency orders; they were merely relaxed for a specified period of time,” Keshishian said. “In the end, all of our policies should balance business interests along with community impacts.”
By the numbers
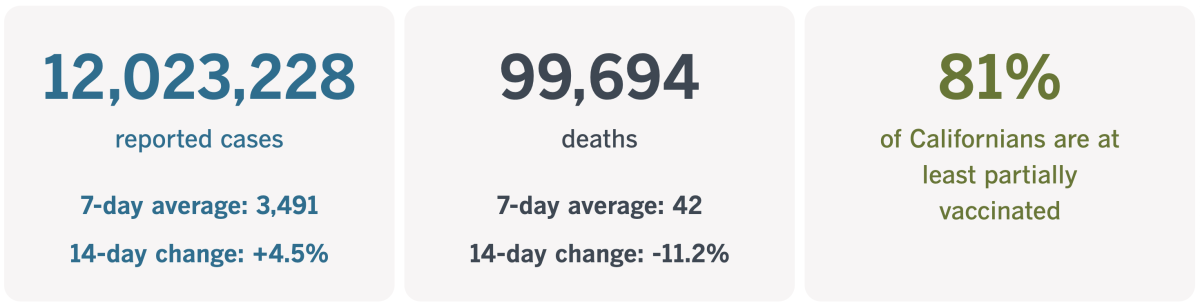
California cases and deaths as of 6:35 p.m. on Tuesday:
Track California’s coronavirus spread and vaccination efforts — including the latest numbers and how they break down — with our graphics.
About those emergency use authorizations ...
It’s not often the pandemic sends us a pleasant surprise. But I’ve got one this week, and it’s about emergency use authorizations. These are the expedited OKs the U.S. Food and Drug Administration has granted for vaccines, medicines, test kits and devices when the country is facing a health emergency.
In normal times, it can take years for a new medical product to come to market. The company developing it must generate exhaustive evidence that the product is safe and effective, a task that usually involves several rounds of clinical trials. The resulting data are then analyzed in great detail before the FDA decides whether to approve it. A study published in JAMA Internal Medicine found that between January 2010 and June 2020, the median time it took to develop a new vaccine was eight years, followed by another year of FDA review.
Those timelines don’t work during a pandemic. During the height of the initial COVID-19 wave, in the spring of 2020, more than 2,100 Americans were succumbing to the disease each day. The country needed vaccines, tests and drugs, and it needed them fast.
Thanks to the EUA process, all kinds of useful things rapidly made their way to the public, some in just a few months. The COVID-specific antiviral remdesivir was authorized for emergency use on May 1, 2020. The initial COVID-19 vaccines from Pfizer-BioNTech and Moderna got their first EUAs in December 2020.
But like the L.A. Al Fresco program, emergency use authorizations weren’t designed to last forever.
“EUAs are effective until the emergency declaration ends,” the FDA explains in a consumer update posted on its website.
In some quarters, that “emergency declaration” was understood to be a reference to the public health emergency that was initially declared by then-Secretary of Health and Human Services Alex Azar on Jan. 31, 2020, and has been renewed more than a dozen times since. That declaration was made possible by the Public Health Service Act, and it gave the government authority to conduct and pay for research “into the cause, treatment, or prevention” of COVID-19, the FDA says.
To others, the “emergency declaration” the FDA seemed to be referring to was Proclamation 9994, which was issued under the National Emergencies Act by President Trump in March 2020. This is the emergency that President Biden intends to wind down May 11.
In actuality, the FDA was talking about a third emergency declaration made possible by the Food, Drug and Cosmetic Act. That’s the law that established the FDA in 1938, and it says that if the secretary of the Department of Health and Human Services declares that “circumstances exist to justify it,” the agency can fast-track the availability of badly needed medicines, vaccines and devices through emergency use authorization.
The declaration that the FDA would invoke its powers to issue EUAs was published April 1, 2020, in the Federal Register. It will remain in effect until the HHS secretary’s emergency declaration is withdrawn, Lawrence Gostin, an expert on public health law at Georgetown University, told my colleague Melissa Healy.
The upshot is that I was wrong last week when I said emergency use authorizations will expire May 11. (Sincere thanks go out to the eagle-eyed reader who alerted me to this.)
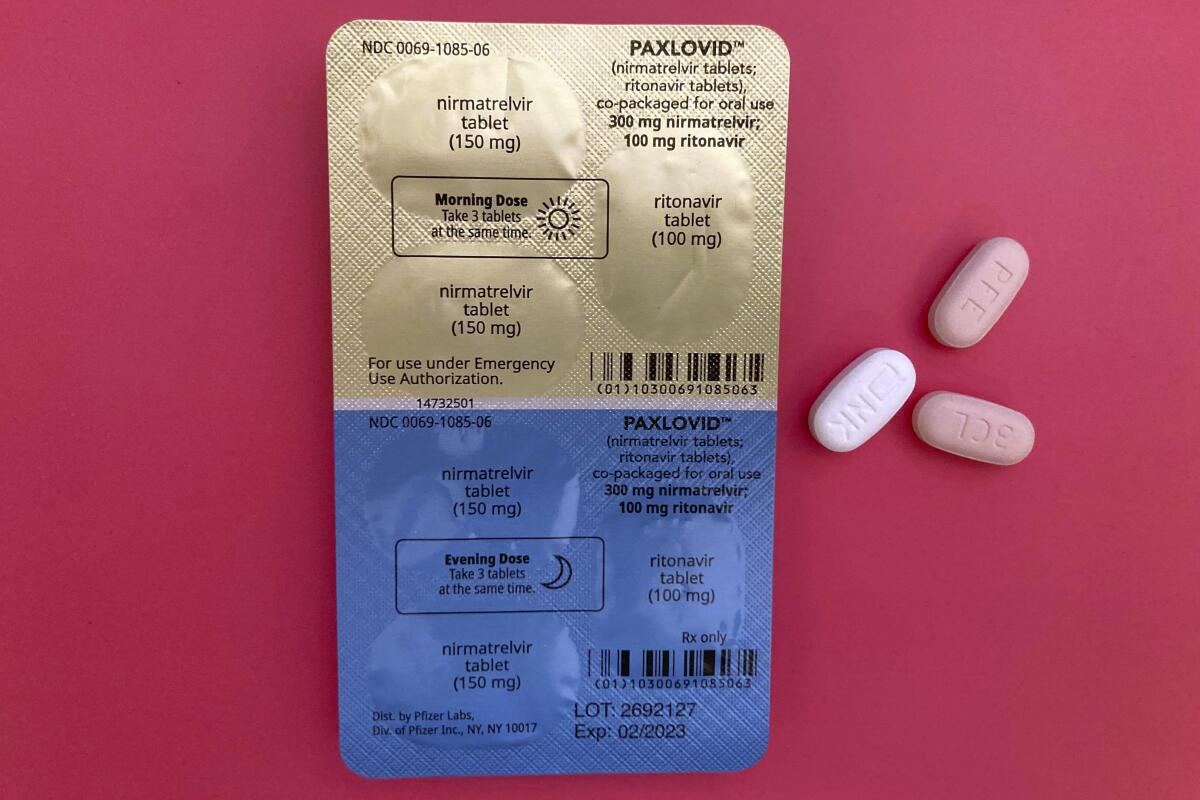
Paxlovid, the workhorse COVID-19 antiviral that is currently available under EUA, will still be on pharmacy shelves to reduce patients’ risk of becoming seriously ill. The same goes for other essential products that are not (yet) FDA-approved, including the new(ish) bivalent boosters that target Omicron and the non-mRNA vaccine from Novavax.
That should be welcome news, considering that only a handful of pandemic items have received full FDA approval. They include the original formulations of the vaccines from Pfizer-BioNTech (Comirnaty) and Moderna (Spikevax), but only for certain age groups (people 12 and older for the former, and adults 18 and up for the latter).
The list also includes remdesivir (trade name Velkury) and two other drugs for hospitalized patients, Actemra (tocilizumab) and Olumiant (baricitinib).
The cost of obtaining familiar items may change after May 11. Vaccines will remain free until the roughly 113 million doses already purchased by the federal government are gone. After that, the Affordable Care Act and other laws will ensure that health plans continue to make the shots available to their customers without a co-pay, but they may well find themselves footing the bill indirectly through higher premiums.
Paxlovid will also be free until Americans use up the 24 million courses the federal government has left. What happens after that depends on whether Congress decides to allocate money to buy more.
California’s vaccination progress
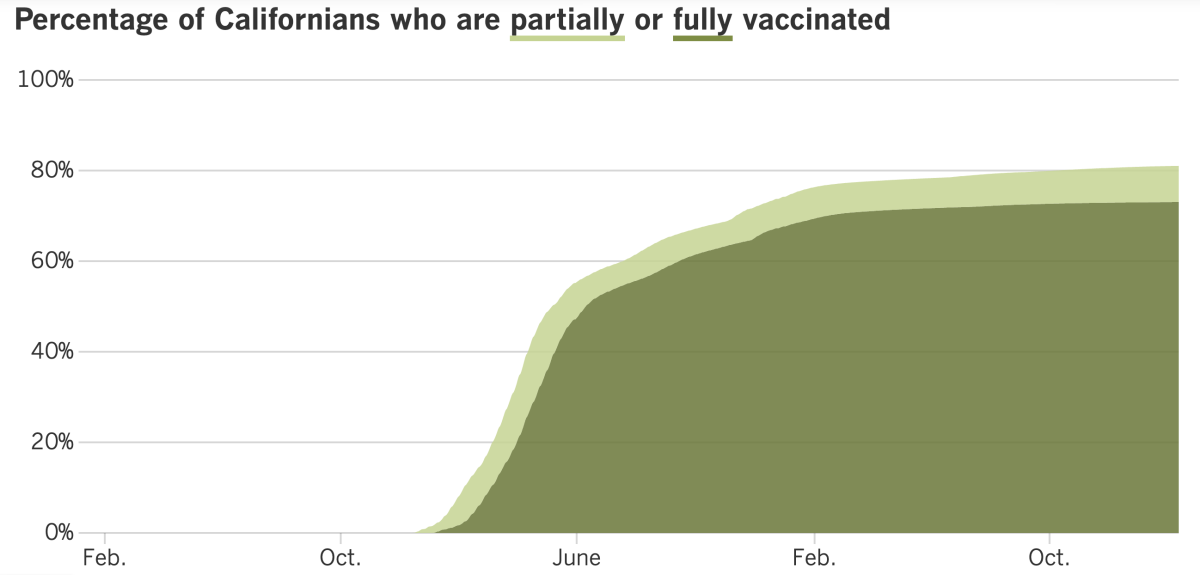
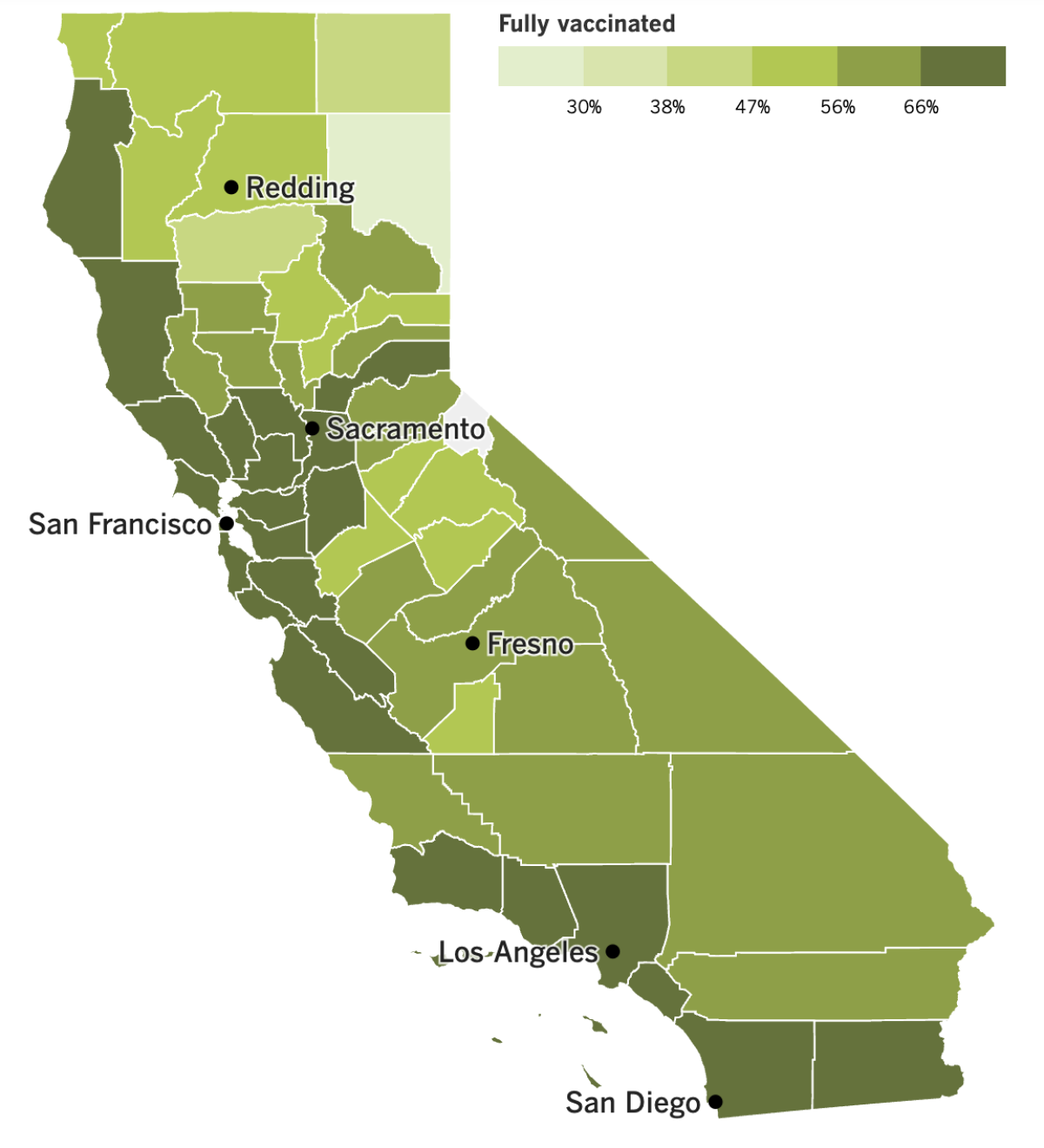
See the latest on California’s vaccination progress with our tracker.
Your support helps us deliver the news that matters most.
In other news ...
It’s been awhile since we’ve invoked the term “grim milestone,” but the occasion calls for it: The number of reported coronavirus cases in California now exceeds 12 million.
That figure would have seemed unthinkable early in the pandemic, when we struggled to believe that the COVID-19 forecasting tool from the Institute for Health Metrics and Evaluation was accurate in projecting that the number of cases could reach 1 million.
Odds are the milestone infection was caused by the Omicron subvariant known as XBB.1.5. It’s believed to be the most infectious version of the SARS-CoV-2 coronavirus to date and accounts for about 75% of coronavirus specimens circulating throughout the U.S. In the region that includes California, an estimated 57% of circulating specimens are XBB.1.5.
As far as milestones go, this is certainly not the grimmest. Becoming infected with the coronavirus isn’t as scary a prospect as it used to be. Most of us have some protection, thanks to vaccination, past infection or both. And if we do get sick, doctors have a handle on how to make us well without time in a hospital.
“We do have more tools now than we’ve had at any point during the pandemic,” said Los Angeles County Public Health Director Barbara Ferrer.
That said, getting COVID-19 isn’t as benign as getting the flu. About 453 people nationwide — and 42 in California — are dying of COVID-19 each day. To put that in perspective, even in an extremely bad flu season, the number of deaths nationwide averages 142 per day.
A grimmer milestone is on California’s horizon. As of Tuesday, the state has recorded 99,694 COVID-19 deaths, according to our tracker. If present trends continue, we’ll hit the 100,000 mark in the next week or so.
The 12 millionth official coronavirus infection was tallied late last week. As of Tuesday, the count is 12,023,228. Both figures are almost certainly lower than the actual number of infections in the state, considering that many cases were never confirmed in the early days of the outbreak and that many cases now identified through at-home testing are not reported to authorities.
But even this undercount represents a figure that’s larger than the populations of all but five states.
Here’s another big number: 152,000. That’s how many children appear to have gone missing from California public schools as a result of the pandemic, according to new research from Stanford University and the Associated Press.
The study examined enrollment figures in the 2019-20, 2020-21 and 2021-22 school years. The state’s school-age population shrank by about 96,000 during that time, according to census data. In addition, the total number of students enrolled in private schools increased by 9,500, and the number of students being home-schooled rose by 14,000. Together, they add up to 119,500 students.
But the overall enrollment decline in the state’s public schools is around 271,000. What happened to the remaining 152,000 students is unknown.
One likely explanation is that some of those students moved to Nevada, Arizona, Texas or another of the 29 states for which enrollment data were not available, so they could not be tracked down. (Only 21 states and the District of Columbia were included in the Stanford/AP analysis.) The report was also unable to track children who moved from California to Mexico.
Another factor is that many families declined to enroll youngsters in kindergarten when distance learning was the only option. State officials have previously said decisions like these helped explain why public school enrollment fell by more than 110,000 last year and by about 160,000 the year before that.
Even after taking these and other trends into account, the numbers suggest that many public students just stopped going to school, according to Thomas Dee, an education researcher at Stanford who wrote the report.
A spokeswoman for the California Department of Education said “the methodology and data used [in the report] does not present a complete picture.” But Bruce Fuller, an education professor at UC Berkeley, said “the findings are sound and eye-popping.”
In other news, the number of California counties whose COVID-19 community levels are classified as “medium” is lower than it was a week ago. Credit for that goes to San Diego and Imperial counties, which moved to the “low” category. As a result, the entire southern part of the state is colored green on the latest COVID-19 community level map from the Centers for Disease Control and Prevention.
Sacramento is now the state’s largest county classified as “medium.” It is joined by neighbors El Dorado, Placer, Solano, Yolo, Napa and Lake, as well as Merced, Stanislaus, Tuolumne and Mariposa in the central part of the state.
The CDC says new cases in Los Angeles County rose more than 16% week over week. On the plus side, COVID-19 deaths fell 19%.
And speaking of the CDC, the agency released a disturbing report Monday detailing the pandemic’s toll on the mental health of U.S. teens — especially for girls and LGBTQ youth.
Surveys of more than 17,000 high school students in the fall of 2021 revealed that nearly 60% of teen girls reported “persistent feelings of sadness or hopelessness.” Nearly 1 in 5 said they’d been victims of rape or another form of sexual violence in the previous year, and 30% said they seriously considered suicide, a rate twice as high as for teen boys.
Among LGBTQ students, almost half said their mental health was “poor,” and a similar fraction said they had seriously contemplated suicide. Across teens of all demographic groups, nearly one-third said they struggled with persistent sadness or hopelessness, an increase over prior years.
Kathleen Ethier, director of the CDC’s adolescent and school health division, said the survey results were the most “devastating” she’s seen in 30 years. “There’s no question young people are telling us they are in crisis,” she said. “The data really call on us to act.”
And finally, COVID-19 vaccines have been fully integrated to the CDC’s official immunization schedules for both children and adults. The new charts recommend a two- or three-dose primary series and a booster starting at 6 months of age, and the advice remains the same all the way up to age 65 and beyond.
Until last week, COVID-19 vaccines were listed in a separate box. Now they rate their own row on the charts, appearing between shots for inactivated poliovirus and influenza on the one for children and adolescents and at the very top of the chart for adults. The change is a way of normalizing them.
The updated immunization schedules also include advice for people who are immunocompromised, along with special guidance for the roughly 19 million Americans who received the Johnson & Johnson vaccine. Situations that would warrant delaying or skipping the vaccines are explained as well.
As of Tuesday, fewer than 16% of Americans are considered up to date with their COVID-19 vaccinations, according to the CDC.
Your questions answered
Today’s question comes from readers who want to know: Should I be worried about the XBB.1.5 variant?
When XBB.1.5 emerged last fall in New York, it earned the nickname “Kraken” for its intimidating mix of mutations. But at this point, it’s safe to breathe easy.
The subvariant is a descendant of two other versions of Omicron, BA.2.10.1 and BA.2.75. Each is adept at binding to cells, the first step in an infection. Blending their talents in a new subvariant helped make XBB.1.5 “the latest and greatest and most infectious variant,” said USC virologist Paula Cannon.
XBB.1.5 also inherited its parents’ ability to evade the immunity gained through vaccination or a past infection. It isn’t susceptible to monoclonal antibody therapies for the same reason.
This combination of traits helped XBB.1.5 quickly dominate the coronavirus landscape in the northeastern United States last year. Fears that the strain would rapidly take over elsewhere didn’t come to pass, but after several months of steady growth, it now accounts for 74.7% of coronavirus specimens in circulation nationwide, the CDC estimates. In the region that includes California, Arizona, Nevada, Hawaii and other Pacific islands, the strain is believed to be responsible for 56.9% of coronavirus infections.
Fortunately, XBB.1.5’s spread from the Northeast has not been accompanied by a surge in hospitalizations. Studies of the subvariant suggest it doesn’t cause more serious illness than its predecessors, nor is it particularly resistant to Paxlovid.
And despite its extreme transmissibility, XBB.1.5 hasn’t caused official case rates to spike in L.A. County.
“While new strains always have the potential to drive surges in cases, to date, we’ve not seen a major increase in cases associated with XBB.1.5.” Ferrer said.
The pandemic in pictures
The tweet pictured above was sent by the FDA. It’s nearly a year and a half old, but it’s arguably the most successful message ever sent from the agency’s Twitter account. To date, it has racked up more than 111,000 likes and nearly 50,000 retweets and been quoted more than 22,000 times.
That tweet is emblematic of the FDA’s efforts to fight misinformation about COVID-19 on social media — a problem that has grown larger since Twitter stopped trying to muzzle false or misleading claims. But calling out lies on social media is more difficult than it might seem, since the very act of repeating a false statement tends to reinforce it.
For Dr. Robert Califf, who took over as FDA commissioner a year ago, fighting misinformation and disinformation is a public health priority. The agency has staffers devoted to creating memes and other catchy content that can stand up for science on social media.
“Historically, the FDA has been able to put out a statement that would be transmitted to doctors, the medical establishment and then the patients,” Califf said. “We’re now in an era where FDA puts out a statement, and then there’s 24-by-7 alternative information that needs to be dealt with.”
You can read more about the FDA’s efforts to make science go viral.
Resources
Need a vaccine? Here’s where to go: City of Los Angeles | Los Angeles County | Kern County | Orange County | Riverside County | San Bernardino County | San Diego County | San Luis Obispo County | Santa Barbara County | Ventura County
Practice social distancing using these tips, and wear a mask or two.
Watch for symptoms such as fever, cough, shortness of breath, chills, shaking with chills, muscle pain, headache, sore throat and loss of taste or smell. Here’s what to look for and when.
Need to get a test? Testing in California is free, and you can find a site online or call (833) 422-4255.
Americans are hurting in various ways. We have advice for helping kids cope, as well as resources for people experiencing domestic abuse.
We’ve answered hundreds of readers’ questions. Explore them in our archive here.
For our most up-to-date coverage, visit our homepage and our Health section, get our breaking news alerts, and follow us on Twitter and Instagram.



