U.S. states build stockpiles of malaria drug touted by Trump
- Share via
SALT LAKE CITY — State and local governments across the United States have obtained more than 30 million doses of a malaria drug touted by President Trump to treat patients with COVID-19, despite warnings from doctors that more research is needed.
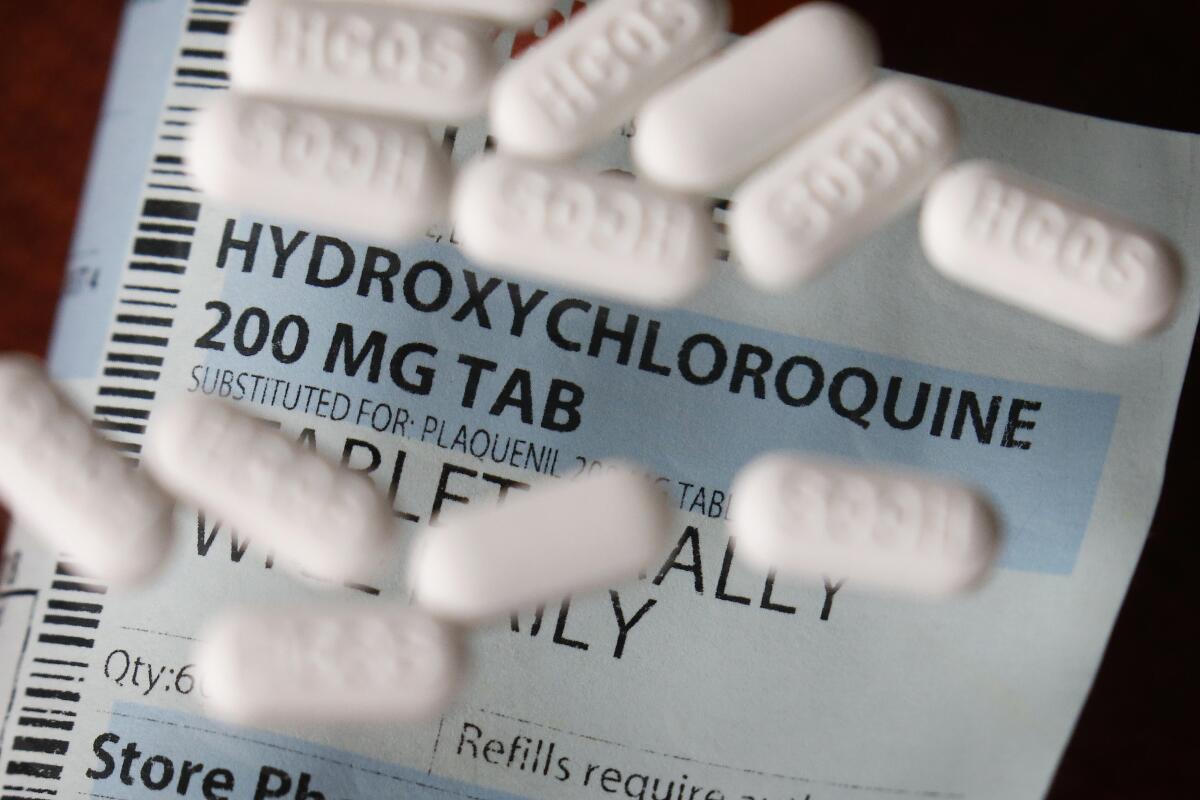
At least 22 states and Washington, D.C., secured shipments of the drug, hydroxychloroquine, according to information compiled from state and federal officials by the Associated Press. Sixteen of those states were won by Trump in 2016, although five of them, including North Carolina and Louisiana, are now led by Democratic governors.
Supporters say having a supply on hand makes sense in case the drug is shown to be effective against the pandemic that has devastated the global economy and killed nearly 200,000 people worldwide, and to ensure a steady supply for people who need it for other conditions like lupus.
But health experts worry that having the drug easily available at a time of heightened public fear could make it easier to misuse it. The U.S. Food and Drug Administration on Friday warned doctors against prescribing hydroxychloroquine for treating COVID-19 outside of hospitals or research settings because of reports of serious side effects, including dangerous irregular heart rhythms and death among patients.
It’s the latest admonition against the drug that Trump mentioned 17 times in various public appearances, touting its potential despite his own health advisers telling him it is unproved.
Oklahoma spent $2 million to buy the drugs, and Utah and Ohio have spent hundreds of thousands on purchases. The rest of the cities and states received free shipments from drug companies or the U.S. government over the last month. Ohio received a large donation from a local company.
Hydroxychloroquine, the drug President Trump hailed as a coronavirus killer, had no beneficial effect for COVID-19 patients in two controlled trials.
Several states, including New York, Connecticut, Oregon, Louisiana, North Carolina and Texas received donations of the medication from a private company based in New Jersey called Amneal Pharmaceuticals. Florida was given 1 million doses from Israeli company Teva Pharmaceutical Industries.
The Federal Emergency Management Agency has sent 19 million doses of hydroxychloroquine to 14 cities, including Philadelphia, Baltimore and Washington, D.C., from the federal government’s national stockpile, a source that also provided South Dakota and California with supplies. The U.S. government received a donation of 30 million doses from Swiss drugmaker Novartis on March 29 to build up the stockpile, which does not normally stock the drug.
“If he [Trump] hadn’t amplified the early and inappropriate enthusiasm for the drug, I doubt if the states would have even been aware of it,” said Dr. Kenneth B. Klein, a consultant from outside of Seattle who has spent the last three decades working for drug companies to design and evaluate their clinical trials.
Klein said it’s understandable that government and health officials looked into hydroxychloroquine — which is approved for treating malaria, rheumatoid arthritis and lupus — as a possible remedy during a frightening pandemic, but the time and energy has been misspent. The potential side effects are worrisome, especially because many coronavirus patients already have underlying health conditions, he said.
“The states and the federal government are reacting in light of that fear. But it’s not a rational response,” Klein said.
Doctors can already prescribe the malaria drug to patients with COVID-19, a practice known as off-label prescribing, and many do. Medical and pharmacy groups have warned against prescribing it for preventive purposes. The FDA has allowed it into the national stockpile, but only for narrowly defined purposes as studies continue.
The FDA has issued a warning contradicting President Trump’s advice on drugs to treat COVID-19 after patients experienced heart issues.
Utah Gov. Gary Herbert, a Republican, has previously acknowledged that the drug is “not without controversy” but defended the state’s efforts to build up a supply. As questions mounted Friday, though, he distanced himself from an $800,000 purchase the state made from a local company and said it would be investigated.
Herbert also halted a plan to spend $8 million more to buy 200,000 additional treatments. “The bottom line is, we’re not purchasing any more of this drug,” he said.
Other states have received it from the federal government. South Dakota, with a population of 885,000 people, received 1.2 million doses and is using the drug for a trial as well as doctor-approved prescriptions for COVID-19 patients.
South Dakota Gov. Kristi Noem, a Republican and Trump ally, said earlier this month she pushed the White House to provide enough hydroxychloroquine to give it to every hospitalized person, others who are vulnerable to the coronavirus and “frontline” healthcare workers. As of Tuesday, 200 people in South Dakota were being treated with the drug, according to Sanford Health.
It is one of several states that say they are using some of the doses for clinical trials to assess whether the drug has benefits for COVID-19 patients.
Many states, however, have opted to steer clear over concerns about side effects and lingering questions about the drug’s effectiveness. At least one of those states, Tennessee, is led by a Republican governor. The state’s Department of Health sent a letter warning against using the drug or hoarding it.
A government scientist felt pressure to approve a research contract for a lab investigating hydroxychloroquine, a drug Trump has touted as a coronavirus cure.
“We were seeing a flood of inappropriate prescribing and hoarding, quite frankly,” Health Commissioner Lisa Piercey told reporters.
Kansas health director Dr. Lee Norman said the state has no plans to buy the drug because evidence is lacking that it helps treat COVID-19.
Most states aren’t paying for the drug. It’s not clear why Utah didn’t get it from the federal reserve or a donation from a business like Amneal Pharmaceuticals.
News releases from state governments show that New Jersey-based Amneal has sent millions of doses of the drug free of cost to states, including 2 million to New York and 1 million to Texas. A company spokesperson declined to provide a list of donations or answer other questions from the Associated Press.
Pharmaceutical companies can often manufacture pills they already make fairly cheaply. The donations may have been made to earn good publicity while setting the company up to make future sales if hydroxychloroquine ends up being a reliable treatment for the virus, Klein said.
Controversy has swirled around the drug since Trump started promoting it March 19 in the White House briefing room.
He mentioned the drug in briefings through April 14, and the White House distributed press releases praising Trump’s efforts to stockpile it for use in areas of the country hard-hit by the novel coronavirus. But for the past week, as studies have shown mixed or even harmful results, Trump has gone silent on the drug.
Asked about it Thursday, Trump said he hadn’t heard of a study done at U.S. veterans hospitals with preliminary results that showed no benefit and rejected the notion he had stopped promoting hydroxychloroquine as a cure.
“I haven’t at all. I haven’t at all,” Trump said. “We’ll see what happens.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.