Polycystic Kidney Disease: Causes, Treatments, and What’s Ahead
- Share via
Key Facts
- PKD is mainly caused by mutations in the PKD1 and PKD2 genes, affecting polycystin proteins.
- Disrupted signaling pathways, like cAMP and vasopressin, are major drivers of cyst growth.
- Tolvaptan is currently the only approved treatment that slows PKD progression.
- Clinical guidelines emphasize genetic counseling and personalized monitoring.
- New research calls for safer treatments and patient-centered outcome measures.
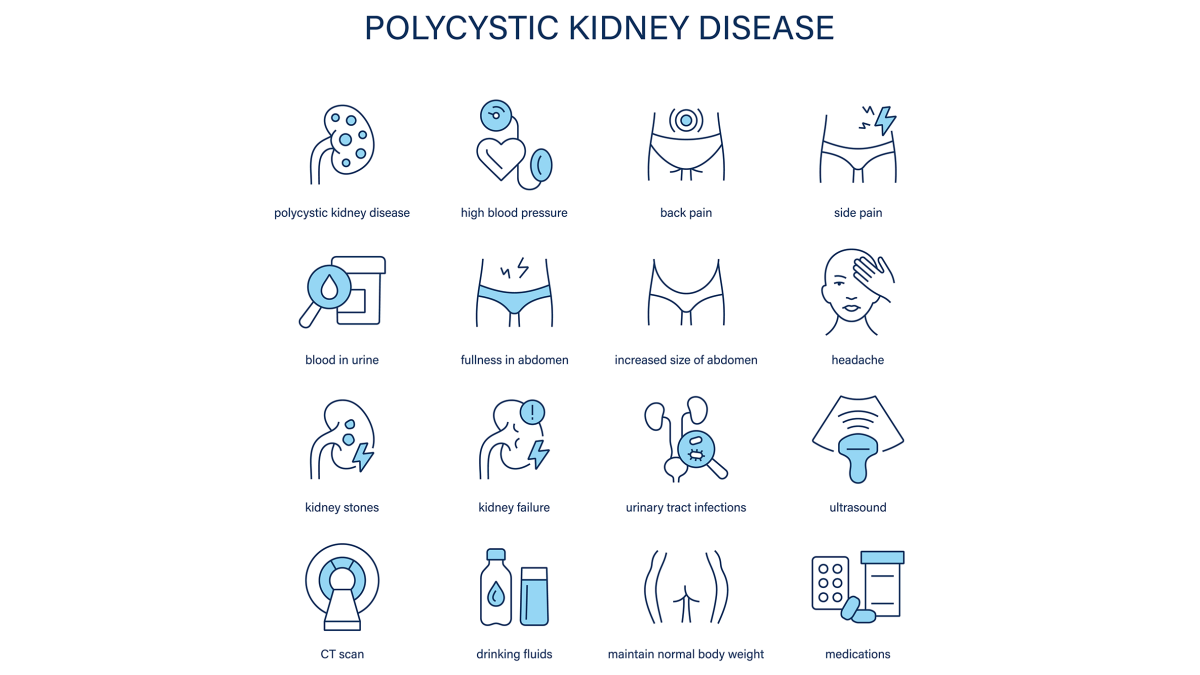
Polycystic Kidney Disease (PKD) is a progressive inherited disorder that causes many cysts, not just fluid filled cysts, to form in the kidneys and ultimately impair their function. PKD is a genetic disease and genetic disorder caused by gene mutations passed from biological parents to their children.
There are two main types of PKD: autosomal dominant PKD (ADPKD) which is the most common and autosomal recessive PKD. The disorder affects men and women equally. Over time these cysts can grow, replace normal kidney tissue and lead to end stage kidney disease (ESKD).
Risk factors for PKD include having an affected parent as the condition is inherited and other genetic variables. PKD can also lead to serious complications beyond kidney failure such as pre-eclampsia and aneurysms.
As we learn more about the genetic and molecular basis of PKD – particularly ADPKD – research is starting to shape more targeted treatments and patient centered care models.
Table of Contents
- Genetic and Molecular Mechanisms
- Intracellular Signaling Pathways and Therapeutic Targets
- Clinical Practice and Guidelines
- Patient-Centered Outcomes and Research Priorities
- Gaps in Knowledge and Future Directions
- Closing Thoughts
- References
Genetic and Molecular Mechanisms
PKD is driven by mutations in two genes: PKD1 and PKD2 which encode the proteins polycystin-1 and polycystin-2. These proteins are crucial for the structure and function of kidney tubules. When they malfunction kidney cells start to divide abnormally and secrete fluid leading to cyst formation and kidney enlargement.
Autosomal recessive PKD (also known as autosomal recessive polycystic kidney disease) is a rarer form of PKD caused by a genetic fault that occurs when both parents carry the abnormal gene and pass it on to their child.
According to a 2014 review in Wiley Interdisciplinary Reviews: Developmental Biology [1] polycystin-1 is a mechanoreceptor – essentially a sensor that helps kidney cells respond to fluid flow. A 2004 study deepens this understanding by showing polycystin-1’s role in complex cellular signaling [2].
More recent research in Physiological Reviews (2025) looks at the primary cilium – a tiny antenna-like structure on kidney cells. This cilium helps sense mechanical changes in fluid flow and interacts with polycystins and another protein fibrocystin all of which are essential in preventing cyst development [4].
Disruption of normal development of the kidneys and liver is a hallmark of autosomal recessive PKD as the genetic fault interferes with the organs’ typical growth and function. While the exact mechanics are still being studied it’s clear that disturbances in this cellular machinery is at the heart of PKD pathology.
Genetic testing can identify mutations in PKD related genes such as PKD1, PKD2 or PKHD1 and help with diagnosis and family planning. A genetic counselor can help families understand inheritance patterns, the risks associated with autosomal recessive PKD and guide them through genetic testing and family planning decisions.
Intracellular Signaling Pathways and Therapeutic Targets
Beneath the genetic mutations are a set of signaling pathways that drive cyst growth. One key player is cyclic AMP (cAMP) which increases fluid secretion and cell proliferation; increased cAMP levels cause cysts to grow in the kidneys.
Other important pathways include epidermal growth factor (EGF) and AMP-activated protein kinase (AMPK) as noted in a 2021 review from Biochemical Society Transactions [5]. These molecules act like traffic signals for cell activity – when their function goes awry cysts can grow unchecked.
Another major breakthrough is on vasopressin receptors, especially V2. The drug called tolvaptan, currently the only FDA approved disease modifying therapy for ADPKD works by blocking these V2 receptors to reduce cAMP production and slow cyst expansion.
For ADPKD patients the drug tolvaptan can slow the rate at which cysts grow and delay disease progression. A 2025 study in the American Journal of Physiology confirms that blocking other receptors (V1a, V1b) doesn’t provide added benefit refining our understanding of this treatment’s specificity [11].

Clinical Practice and Guidelines
Modern PKD care is about early diagnosis, genetic counseling and personalized treatment. To diagnose PKD imaging tests such as ultrasound, computed tomography scan and MRI scans are used to detect and monitor cyst development. The 2020 Chinese clinical practice guidelines are a comprehensive resource for clinicians covering everything from risk stratification to long term monitoring and intervention strategies [3].
These guidelines build on earlier frameworks such as the 2018 Nature Reviews: Disease Primers article which bridges cellular biology with real world clinical application [6]. Meanwhile a 2017 review in Comprehensive Physiology helps contextualize both hereditary and sporadic forms of PKD and offers a deeper dive into how and why cysts form [9].
Patient Outcomes and Research Priorities
As treatment advances patient voices are becoming more central to shaping research priorities. A 2025 study in Kidney360 gathered insights from patients, caregivers and healthcare professionals and found that preserving kidney function, improving quality of life and managing related health conditions are top concerns [10].
PKD can have a big impact on mental health. Many patients experience emotional challenges such as depression and anxiety. Addressing these mental health concerns is essential for overall well being.
Healthcare providers play a crucial role in supporting patients by offering resources, guidance and referrals to mental health professionals when needed. Adopting a healthy lifestyle – staying active, reducing stress, quitting smoking and maintaining a healthy weight – can help manage PKD and related health conditions.
This shift towards value based, participatory care is a trend across nephrology – where patients are not just recipients of care but active collaborators in defining meaningful outcomes.
Gaps in Knowledge and Future Directions
Even with these advances there are still many questions:
- What are the exact molecular functions of polycystins and fibrocystin?
- Can we identify early biomarkers to predict which patients will progress rapidly?
- Are there safer alternatives to tolvaptan, especially those that reduce its aquaretic side effects (excessive urination and thirst)?
Research is ongoing to prevent kidney damage and to avoid factors that can make kidney damage worse in PKD. PKD is one of many kidney diseases; for example acquired cystic kidney disease can develop in people with chronic kidney disease especially those on long term dialysis. Prevention of kidney failure is key for PKD patients.
Historical perspectives from the 2009 Annual Review of Medicine and 2013 Minerva Urologica e Nefrologica show just how far we have come and how far we have to go [7] [8]. These reviews highlight the need for therapies that not only slow cyst growth but also reverse or repair the cellular defects that underlie PKD.
Closing Thoughts
Polycystic Kidney Disease is a complex condition rooted in genetic and cellular abnormalities that are slowly being uncovered by modern science. Advances in molecular biology, imaging and drug development have improved how the disease is diagnosed and managed.
But long term success depends on closing the gaps in knowledge, refining the therapies and keeping patients at the centre of both clinical and research efforts.
References
[1] Paul, B. M., & Vanden Heuvel, G. B. (2014). Kidney: polycystic kidney disease. Wiley interdisciplinary reviews. Developmental biology, 3(6), 465–487. https://doi.org/10.1002/wdev.152
[2] Wilson P. D. (2004). Polycystic kidney disease: new understanding in the pathogenesis. The international journal of biochemistry & cell biology, 36(10), 1868–1873. https://doi.org/10.1016/j.biocel.2004.03.012
[3] Writing Group For Practice Guidelines For Diagnosis And Treatment Of Genetic Diseases Medical Genetics Branch Of Chinese Medical Association, Xu, D., & Mei, C. (2020). Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chinese journal of medical genetics, 37(3), 277–283. https://doi.org/10.3760/cma.j.issn.1003-9406.2020.03.009
[4] Boletta, A., & Caplan, M. J. (2025). Physiologic mechanisms underlying polycystic kidney disease. Physiological reviews, 105(3), 1553–1607. https://doi.org/10.1152/physrev.00018.2024
[5] Richards, T., Modarage, K., Malik, S. A., & Goggolidou, P. (2021). The cellular pathways and potential therapeutics of Polycystic Kidney Disease. Biochemical Society transactions, 49(3), 1171–1188. https://doi.org/10.1042/BST20200757
6] Bergmann, C., Guay-Woodford, L. M., Harris, P. C., Horie, S., Peters, D. J. M., & Torres, V. E. (2018). Polycystic kidney disease. Nature reviews. Disease primers, 4(1), 50. https://doi.org/10.1038/s41572-018-0047-y
[7] Harris, P. C., & Torres, V. E. (2009). Polycystic kidney disease. Annual review of medicine, 60, 321–337. https://doi.org/10.1146/annurev.med.60.101707.125712
[8] Czarnecki, P. G., & Steinman, T. I. (2013). Polycystic kidney disease: new horizons and therapeutic frontiers. Minerva urologica e nefrologica = The Italian journal of urology and nephrology, 65(1), 61–68. https://pubmed.ncbi.nlm.nih.gov/23538311/
[9] Ghata, J., & Cowley, B. D., Jr (2017). Polycystic Kidney Disease. Comprehensive Physiology, 7(3), 945–975. https://doi.org/10.1002/cphy.c160018
[10] Mustafa, R. A., Kawtharany, H., Kalot, M. A., Lumpkins, C. Y., Kimminau, K. S., Creed, C., Fowler, K., Perrone, R. D., Jaure, A., Cho, Y., Baron, D., & Yu, A. S. L. (2025). Establishing Meaningful Patient-Centered Outcomes with Relevance for Patients with Polycystic Kidney Disease: Patient, Caregiver, and Researcher Priorities for Research in Polycystic Kidney Disease. Kidney360, 6(4), 573–582. https://doi.org/10.34067/KID.0000000695
[11] Wang, X., Jiang, L., Nanayakkara, K., Hu, J., & Torres, V. E. (2025). Vasopressin V1a and V1b receptor antagonism does not affect the efficacy of tolvaptan in Polycystic Kidney Disease. American journal of physiology. Renal physiology, 10.1152/ajprenal.00350.2024. Advance online publication. https://doi.org/10.1152/ajprenal.00350.2024












