Huntington’s Disease: Genetics, Symptoms, and Hope for the Future

- Share via
Key Facts
- Huntington’s disease is caused by a CAG repeat expansion in the HTT gene.
- Symptoms typically begin between ages 30 and 50 and include motor, cognitive, and psychiatric issues.
- Diagnosis is confirmed through genetic testing and neuroimaging.
- There’s no cure, but symptom management and emerging gene-silencing therapies offer hope.
- A multidisciplinary approach is essential for managing HD’s emotional, physical, and social impacts.
Huntington’s disease (HD) is one of those rare conditions that affects not just the patient but the entire family—medically, emotionally and genetically. This inherited brain disorder causes gradual breakdown of nerve cells especially in the parts of the brain involved in movement, thinking and mood regulation. Although rare, affecting 3 to 7 people per 100,000 globally, its impact is profound and relentless. With no cure in sight, HD is at the center of intense research to understand its molecular roots and develop targeted therapies [1].
Table of Contents
- Huntington Disease Symptoms: Understanding their Genetic Basis and Pathogenesis
- Clinical Features and Huntington’s Disease Symptoms
- Diagnosis and Genetic Testing
- Pathophysiological Insights into Huntington’s Disease
- Therapeutic Strategies for Huntington’s Disease
- Psychosocial and Ethical Considerations
- Closing Thoughts
- References
Huntington Disease Symptoms: Understanding their Genetic Basis and Pathogenesis
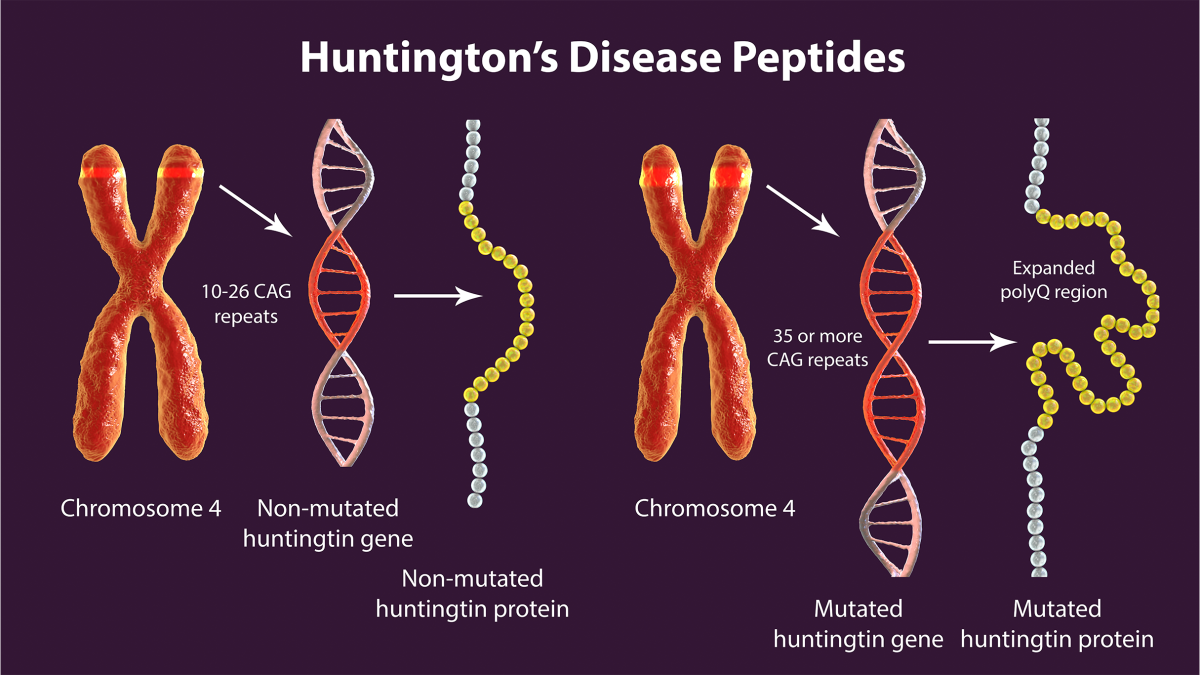
Huntington’s disease is inherited in an autosomal dominant pattern, meaning if a person inherits one copy of the defective gene they will develop the disease. The culprit is a genetic mutation in the hd gene (also known as the huntingtin gene), where a DNA segment—specifically a CAG trinucleotide repeat—is abnormally expanded. Normally this segment is repeated 10 to 35 times. In HD it’s repeated 36 times or more, sometimes even in the 100s [3] [4] [5] [6]. Huntington’s disease is caused by a genetic mutation in the huntingtin gene, specifically the HD mutation involving expanded CAG repeats.
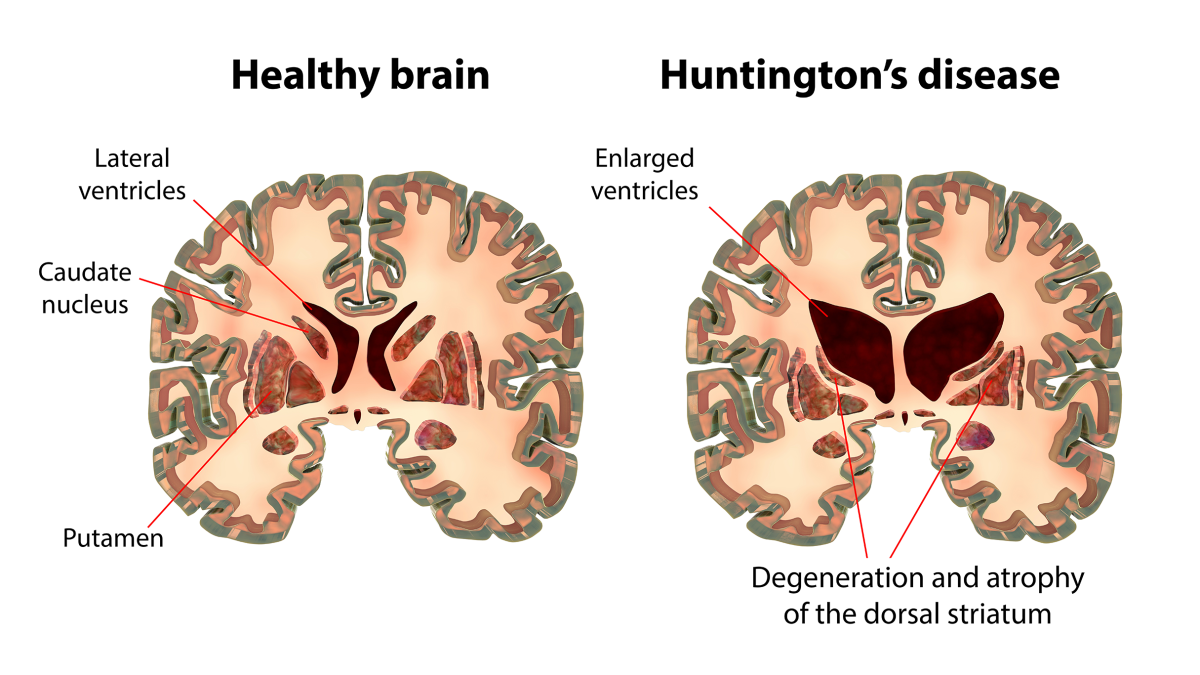
This expanded repeat leads to the creation of a toxic version of a protein called huntingtin which misfolds and accumulates in brain cells. Over time these protein clumps disrupt cellular function and lead to death of neurons—especially in the striatum and cortex, areas responsible for motor control and cognition [4] [5]. The loss of nerve cells in these regions is what causes the symptoms of Huntington’s disease. Different genetic variants can influence the age of onset and progression of the disease.
Researchers have identified two distinct disease phases: an early phase where the brain seems to compensate for the damage and a later phase where symptoms are more visible and rapid neurodegeneration [7]. Understanding the disease process at the molecular level is key to developing new treatments.
Clinical Features and Huntington’s Disease Symptoms
Huntington’s disease symptoms usually emerge between 30-50 years and progress over 15-20 years. The classic triad of symptoms includes:
- Motor symptoms: These are often the first to appear. Patients may develop involuntary jerking movements called chorea, muscle rigidity, slurred speech and problems with balance or walking. Huntington’s disease chorea is a hallmark feature of the condition. Huntington’s chorea is a type of movement disorder characterized by involuntary, dance-like movements. These uncontrolled movements can interfere with daily activities and increase the risk of complications. Physical symptoms like chorea and coordination issues are visible signs of the disease. Huntington’s disease is classified under movement disorders due to its impact on voluntary and involuntary movements [2].
- Cognitive decline: This involves difficulties with attention, memory, planning and decision-making. Many patients struggle with multitasking and organizing information. Cognitive and behavioral symptoms are common, affecting mental processes and emotional regulation.
- Psychiatric disturbances: Depression, irritability, apathy and even psychosis can occur. These behavioral symptoms may include mood swings and often contribute significantly to disability. Mood swings are a frequent feature, reflecting emotional instability.
The range of disease symptoms includes symptoms of HD and symptoms of Huntington’s disease, covering motor, cognitive and behavioral changes throughout the course of the illness.
Research in 2025 has shown that changes in how the striatum connects with the hippocampus may explain some of the memory difficulties HD patients experience, especially those involving spatial awareness [10]. Also worth noting are lesser-known symptoms like loss of smell in advanced disease stages [11] and subtle sex-based differences in disease expression. For example, male patients may have lower levels of 17β-estradiol and reduced number of DARPP-32+ neurons, which may influence disease severity [12]. Other symptoms like sleep disturbances and weight loss may also appear as the disease advances.
As the disease progresses symptoms worsen over time with increasing severity of movement disorders, cognitive decline and behavioral changes.
Motor symptoms especially uncontrolled movements and balance problems can lead to physical injury from falls or accidents, further impacting quality of life.
Juvenile Huntington’s disease is a rare form that affects children and adolescents, often presenting with unique features like seizures and more rapid progression compared to adult-onset cases.
Diagnosis and Genetic Testing
Huntington’s disease diagnosis is confirmed by a genetic test showing 36 or more CAG repeats in the HTT gene. However, the diagnostic process also involves:
- A detailed medical history and family medical history
- Neurological exam and clinical observation of movement, mood and cognitive symptoms
- Brain imaging, such as MRI scans, which can show atrophy in the caudate nucleus and putamen
Presymptomatic genetic testing is available for individuals without known family history of HD. While this can provide clarity, it raises ethical challenges, especially around mental health support and family planning [9].
Pathophysiological Insights into Huntington’s Disease
Beyond the faulty gene itself, scientists have found a cascade of biological disruptions that drive HD. Imaging studies show that HD affects brain structure and function, leading to progressive changes in key brain regions and chemical systems:
- Mitochondrial dysfunction: Neurons in HD use energy less efficiently, making them more prone to damage.
- Oxidative stress: Free radicals accumulate and harm cellular components.
- Transcriptional dysregulation: The mutant huntingtin protein interferes with normal gene expression.
- Impaired protein degradation: The system responsible for clearing out damaged proteins becomes overwhelmed.
- Neuroinflammation: Even immune cells outside the brain show abnormal gene expression, indicating systemic involvement [8].
Huntington’s disease affects both physical and mental health, leading to movement disorders, cognitive decline and psychiatric symptoms that worsen over time.
Each of these changes leads to neuronal death, providing multiple targets for therapeutic intervention. Individuals who inherit the genetic mutation will develop Huntington’s disease.
Therapeutic Strategies for Huntington’s Disease
There’s no cure for HD yet, but several treatments can help manage symptoms:
- Chorea: Medications like tetrabenazine and deutetrabenazine reduce involuntary movements.
- Psychiatric symptoms: Antidepressants and antipsychotics are commonly used.
- Supportive care: Physical therapy, occupational therapy, and speech therapy are important, and speech therapists specifically help people with Huntington’s disease manage speech, communication, and swallowing difficulties.
More exciting, however, are the experimental approaches in development:
- Gene-silencing therapies: Antisense oligonucleotides (ASOs) are designed to reduce production of the mutant huntingtin protein.
- Neuroprotective agents: These aim to shield neurons from oxidative damage and inflammation.
- Cell and gene therapies: Still in early trials, these strategies hold promise for halting or even reversing disease progression [3] [6].
- Clinical trials are essential for testing new treatments and interventions, and participation by people with Huntington’s disease helps advance research and improve future care.
For updates on these trials, you can follow progress via resources like HDSA.org, the ClinicalTrials.gov HD portal, or the European Huntington’s Disease Network.
People with Huntington’s disease benefit from tailored care and support, and their involvement in research and clinical trials is crucial for developing better therapies.
Psychosocial and Ethical Considerations
Living with Huntington’s isn’t just about managing symptoms—it’s about navigating a complex emotional and social landscape. Many patients grapple with:
- The psychological weight of a known genetic risk
- Family planning decisions
- Loss of independence as symptoms progress
- Financial and caregiving stress
Family members are often deeply involved in caregiving, emotional support, and making important decisions throughout the course of the disease.
Because of this, a team-based approach to care is critical. Neurologists, psychiatrists, genetic counselors, social workers, therapists, and genetic counseling services all play a part in supporting HD patients and their loved ones. A genetic counselor is a healthcare professional who guides patients through the genetic testing process, explains inheritance patterns, and answers questions about the benefits and risks of testing.
Closing Thoughts
Huntington’s disease is a devastating diagnosis, but the landscape is slowly shifting. Thanks to advances in genetic research, brain imaging, and experimental therapies, we’re moving closer to more personalized and effective treatments. Until then, early diagnosis, supportive care, and active participation in research remain key to improving quality of life for those affected by HD.
References
[1] Stoker, T. B., Mason, S. L., Greenland, J. C., Holden, S. T., Santini, H., & Barker, R. A. (2022). Huntington’s disease: diagnosis and management. Practical neurology, 22(1), 32–41. https://doi.org/10.1136/practneurol-2021-003074
[2] Walker F. O. (2007). Huntington’s disease. Lancet (London, England), 369(9557), 218–228. https://doi.org/10.1016/S0140-6736(07)60111-1
[3] Kim, A., Lalonde, K., Truesdell, A., Gomes Welter, P., Brocardo, P. S., Rosenstock, T. R., & Gil-Mohapel, J. (2021). New Avenues for the Treatment of Huntington’s Disease. International journal of molecular sciences, 22(16), 8363. https://doi.org/10.3390/ijms22168363
[4] Ghosh, R., & Tabrizi, S. J. (2018). Huntington disease. Handbook of clinical neurology, 147, 255–278. https://doi.org/10.1016/B978-0-444-63233-3.00017-8
[5] McColgan, P., & Tabrizi, S. J. (2018). Huntington’s disease: a clinical review. European journal of neurology, 25(1), 24–34. https://doi.org/10.1111/ene.13413
[6] Bates, G. P., Dorsey, R., Gusella, J. F., Hayden, M. R., Kay, C., Leavitt, B. R., Nance, M., Ross, C. A., Scahill, R. I., Wetzel, R., Wild, E. J., & Tabrizi, S. J. (2015). Huntington disease. Nature reviews. Disease primers, 1, 15005. https://doi.org/10.1038/nrdp.2015.5
[7] Hong, E. P., MacDonald, M. E., Wheeler, V. C., Jones, L., Holmans, P., Orth, M., Monckton, D. G., Long, J. D., Kwak, S., Gusella, J. F., & Lee, J. M. (2021). Huntington’s Disease Pathogenesis: Two Sequential Components. Journal of Huntington’s disease, 10(1), 35–51. https://doi.org/10.3233/JHD-200427
[8] Wolf, B., Schwarzer, A., Côté, A. L., Hampton, T. H., Schwaab, T., Huarte, E., Tomlinson, C. R., Gui, J., Fisher, J. L., Fadul, C. E., Hamilton, J. W., & Ernstoff, M. S. (2012). Gene expression profile of peripheral blood lymphocytes from renal cell carcinoma patients treated with IL-2, interferon-α and dendritic cell vaccine. PloS one, 7(12), e50221. https://doi.org/10.1371/journal.pone.0050221
[9] Rodríguez-Arribas, M., Yakhine-Diop, S. M. S., Pedro, J. M. B., Gómez-Suaga, P., Gómez-Sánchez, R., Martínez-Chacón, G., Fuentes, J. M., González-Polo, R. A., & Niso-Santano, M. (2017). Mitochondria-Associated Membranes (MAMs): Overview and Its Role in Parkinson’s Disease. Molecular neurobiology, 54(8), 6287–6303. https://doi.org/10.1007/s12035-016-0140-8
[10] Glikmann-Johnston, Y., Delagneau, G., Barta, T., Stout, J. C., & Razi, A. (2025). Neural Mechanisms of Object Location Memory in Huntington’s Disease. Movement disorders : official journal of the Movement Disorder Society, 10.1002/mds.30232. Advance online publication. https://doi.org/10.1002/mds.30232
[11] Bylsma, F. W., Moberg, P. J., Doty, R. L., & Brandt, J. (1997). Odor identification in Huntington’s disease patients and asymptomatic gene carriers. The Journal of neuropsychiatry and clinical neurosciences, 9(4), 598–600. https://doi.org/10.1176/jnp.9.4.598
[12] Bode, F. J., Stephan, M., Suhling, H., Pabst, R., Straub, R. H., Raber, K. A., Bonin, M., Nguyen, H. P., Riess, O., Bauer, A., Sjoberg, C., Petersén, A., & von Hörsten, S. (2008). Sex differences in a transgenic rat model of Huntington’s disease: decreased 17beta-estradiol levels correlate with reduced numbers of DARPP32+ neurons in males. Human molecular genetics, 17(17), 2595–2609. https://doi.org/10.1093/hmg/ddn159












